16 EC-IC Bypass Evidence
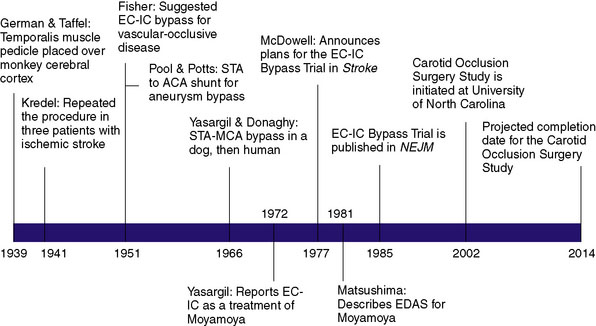
The first description of an operation intended to provide alternative cerebral blood flow (CBF) was given by German and Taffel in 1939, in which a temporalis muscle pedicle was placed over the cerebral cortex in monkeys (Figure 16–1).1 They later subjected both treated and untreated controls to carotid and vertebral occlusion, and reported the monkeys with temporalis pedicles overlying the cortex survived, whereas untreated controls did not. In 1942, Kredel first performed the procedure in three human patients who had suffered ischemic stroke, reporting postoperative clinical improvement in all three patients.2 However, the technique was largely abandoned over the next decade when postoperative angiograms failed to provide evidence of collateral flow through the graft.
C. Miller Fisher shed new light on the concept of re-establishing cerebral perfusion in 1951 when he postulated that extracranial to intracranial (EC-IC) arterial bypass might be performed as a treatment for occlusive vascular disease.3 That same year, Pool and Potts created a superficial temporal artery (STA) to anterior cerebral artery (ACA) shunt during treatment of a giant ACA aneurysm. Although the shunt was successful intraoperatively, postoperative angiogram confirmed the shunt had become occluded.4
The first EC-IC arterial bypass was not described until 1966 when Yasargil and Donaghy successfully performed an STA to middle cerebral artery (MCA) anastomosis in a dog. Shortly thereafter, Yasargil successfully performed the same procedure in a human, providing the neurosurgical community with what was deemed a breakthrough in the treatment of intracranial atherosclerosis. Following Yasargil’s description of the EC-IC arterial bypass, neurosurgeons around the world utilized the procedure with the intent of re-establishing CBF in a variety of cerebrovascular diseases. Surgeons subsequently described bypasses from the occipital and middle meningeal arteries to the MCA, from the occipital artery to the posterior inferior cerebellar artery,5 from the occipital artery to the anterior inferior cerebellar artery (AICA), and from the STA to the superior cerebellar artery.5,6
Over time surgical technique improved, and the most frequently employed bypass procedure became the STA to MCA bypass for the indication of symptomatic internal carotid artery (ICA) or MCA stenosis. However, as was the case with most other surgically treated diseases at the time, there were no extant data to demonstrate that performing the procedure provided patients with any significant short- or long-term benefit. The only early report came in the form of a small retrospective series published by Sundt et al. in 1977, the utility of which was limited by multiple biases. The group reported on a retrospective series of 56 patients who underwent STA-MCA bypass for a wide variety of indications, including TIAs, “orthostatic ischemia,” progressive stroke, and aneurysmal bypass. With regards to graft patency, those who had grafts prior to 1973 had low patency (25%) whereas those operated after 1974 had high patency (95%). The other outcome described was major ischemic stroke or death from the surgery, of which they reported none. The report served more to describe the acceptable safety of the procedure, rather than to examine its efficacy.7
Too often surgical treatment for a particular condition gains enthusiasm and wide use before any clear evidence of effectiveness. This problem has been particularly evident in the field of atherosclerotic vascular disease involving the treatment of its cardiac and cerebral complications. The recent suggestions for extracranial/intracranial arterial anastomosis in the prevention and treatment of stroke have without question a logical basis. This is especially true when atherosclerosis in cerebral vessels lies distal to the surgically accessible portion of the internal carotid artery. Anastomosis of the superficial temporal artery to the middle cerebral artery or one of its branches to improve vertebral circulation in such situations is beginning to be carried out frequently in a number of centers across the United States. In an effort to determine the effectiveness of this therapy 20 major medical centers in the United States and three centers outside the United States have joined together in a collaborative study of this therapy.8
Pending the results of the EC-IC Bypass Study, neurosurgeons published a handful of case reports, series, and commentaries on the appropriate indications, safety, and utility of EC-IC bypass.9–15 In 1983, Chater reported on 400 patients who underwent EC-IC bypass for TIAs and hemodynamic intracranial lesions, with a permanent neurological morbidity rate of approximately 2% and operative mortality of 2.5%, and an ipsilateral postoperative stroke incidence of 0.9% per year after a 55-month follow-up.16 Chater et al. then reported on 105 patients with intracranial ICA stenosis (60% to 98%) and ischemia 1 to 3 months prior to EC-IC bypass. Of these, surgical mortality was 1%, permanent surgical morbidity was 2%, and after 54 months of follow-up, 22 patients had died, of which three were related to stroke; thus the overall late death rate was 4% per year. Although Chater’s reports had begun to examine a more uniform patient population, both of his retrospective series contained no medically treated control group against which the success of surgery could be measured.17
International cooperative study of ec-ic arterial anastomosis
In 1985, nearly 8 years after McDowell’s introduction of the International Cooperative Study of Extracranial-Intracranial Arterial Anastomosis (termed the EC-IC Bypass Trial), the group reported the results of this randomized, prospective trial. Of the 1377 patients enrolled in the trial, 714 (52%) were assigned to medical treatment alone (daily aspirin and aggressive hypertension control) and 663 (48%) were assigned to receive EC-IC bypass in addition to medical therapy. Inclusion and exclusion criteria are presented in Table 16–1.
Table 16–1 Inclusion and Exclusion Criteria for the EC-IC Bypass Trial.
Notably, patients were allowed to enroll in the trial if they had tandem stenosis (two separate stenosis lesions) of the proximal and distal ICA, the proximal lesion being amenable to carotid endarterectomy18 and the distal lesion qualifying for EC-IC bypass. Which surgery was performed first was left to the discretion of the operating surgeons, with the caveat that CEA, if performed first, was to occur at least 30 days prior to randomization in the EC-IC Bypass Trial.
The results of the trial demonstrated that EC-IC bypass provided no significant reduction in major and fatal strokes, ipsilateral strokes, major ipsilateral strokes, or all strokes and deaths combined. Separate analyses in patients with different angiographic lesions did not identify a subgroup with any benefit from surgery. They also reported 30-day surgical mortality and major stroke morbidity rates of 0.6% and 2.5%, respectively. The graft patency rate was reported as 96%, roughly similar to previous series.19
Study Criticism
Furthermore, the bypass study contained no description of patients’ collateral circulation sources into the clinically affected hemisphere. This information was considered critical with the advent of carotid endarterectomy data showing that intraoperative determination of collateral flow by radioactive xenon washout was predictive of subsequent stroke: no infarctions occurred in patients with collateral CBF greater than 40 ml/100 g/min, whereas strokes incidence was 3.4% per year when CBF was less than 25 ml/100 g/min.20 The concept applies to EC-IC bypass in that the ideal candidate would be one with naturally available but insufficient collateral channels, for which the bypass would provide improved blood flow.
Moyamoya Disease: EC-IC Bypass and EDAS
With the advent of angiography, Moyamoya, a disease in children and adults characterized by progressive multiple stenoses of the ICA, MCA, and ACA, was recognized as a physiologic entity distinct from the kind of adult intracranial stenosis heretofore discussed. Moyamoya occlusions result in secondary neovascularization of the lenticulostriate and leptomeningeal circulation, resulting in the characteristic “puff of smoke” appearance of collateral vessels on angiogram. As a consequence of Moyamoya, children typically present with strokes and adults typically present with intracranial hemorrhage related to friable vessels. As bypass procedures emerged, the Moyamoya population was identified as ideal for reconstituting flow for at-risk tissue. Yasargil was the first to report EC-IC bypass for Moyamoya in 1972, and a number of case reports describing STA-MCA bypass followed in the ensuing decades.21–25 The STA-MCA bypass was generally considered the treatment of choice for Moyamoya at the time, though there were a number of patients (children, mostly) whose STA was too small to serve as an adequate pedicle.
In 1981, Matsushima et al. first described the encephaloduroarteriosynangiosis (EDAS), in which the distally intact STA and a strip of galea were mobilized, and simply placed over the cortex and sutured to the dura. The idea of the procedure was to help promote the natural tendency of the disease to develop collateral circulation.26 The procedure was considered simple and safe because the arteries remain intact, requiring no microvascular anastomosis. Matsushima subsequently reported on 50 pediatric and four adult patients, stating that 12-month angiograms showed marked revascularization through the external carotid system.25 Olds and Spetzler also reported early good results with unilateral or bilateral EDAS.27
Although no prospective studies have been performed comparing EDAS to EC-IC bypass or medical treatment, recent case series continue to support its application in the treatment of Moyamoya in both children and adults.28–34 We recently reported long-term outcome in our series of 43 adults treated with EDAS for Moyamoya. Comparing infarct-free survival rates in surgically treated hemispheres and non-surgically treated hemispheres, we found that the 5-year, infarction-free rate was 94% versus 36% in the untreated hemispheres.34
Both indirect and direct revascularization procedures continue to be employed in the treatment of Moyamoya depending on patient characteristics and institutional preference. A large-scale retrospective study was published in Japan in 1997 examining 290 patients with hemorrhagic Moyamoya, including 152 patients who underwent EC-IC bypass and 138 patients who were treated conservatively with medical therapy.35 In the surgical group, 19.1% experienced recurrent hemorrhage, while 28.3% had recurrent hemorrhage in the nonsurgical group. A large prospective trial, the Japan Adult Moyamoya Trial, is now underway in Japan to analyze the efficacy of direct EC-IC bypass for Moyamoya.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree