Chapter 6 EEG-Correlated fMRI in Epilepsy
Current State of the Art
Introduction
EEG-correlated fMRI, commonly referred to as EEG-fMRI, is a magnetic resonance imaging (MRI) technique that uses the simultaneously recorded electroencephalogram (EEG) as a marker of brain activity. EEG is a measure of a neuronal activity, whereas fMRI reflects hemodynamic changes, in particular blood oxygen level–dependent (BOLD) signal, which is the main contrast mechanisms exploited by fMRI. Although it is clear that BOLD signal change is indirectly linked to neuronal activity, it is not a clear relationship, and the nature of this “neurovascular coupling” is not fully understood. The study of the hemodynamic correlates of pathological EEG patterns observed in epilepsy by use of EEG-fMRI has developed since the mid-1990s and indeed provided the original impetus for this development. While the initial motivation for combining the two modalities was to overcome some of the deficiencies in each technique, in particular the problem of EEG source localization (the inverse solution) and the low temporal resolution of fMRI, EEG-fMRI uniquely allows the study of the haemodynamic correlates of spontaneous brain activity and in particular individual interictal discharges and seizures.
Interictal Activity in Focal Epilepsy
Using an interictal epileptic discharge (IED) triggered technique, whereby images were only acquired following the observation of an EEG event of interest and compared voxel by voxel to images acquired following periods of background EEG, initial studies in selected patients with focal epilepsy revealed significant BOLD increases in a large proportion of cases that were reproducible,1 although the statistical tests applied varied widely2–8 (see Table 6-1). It should be noted that the early studies focused on the identification of BOLD signal increases, neglecting the possibility of BOLD decreases, which were subsequently shown to be of considerable interest (see discussion later in this chapter).
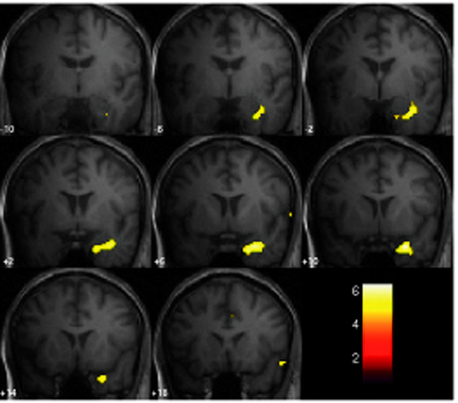
The continuous EEG-fMRI acquisition approach was made possible by the use of EEG processing methods to correct scanning-related artifact9 (see the section on technical aspects at the end of this chapter for further discussion). The analysis of this type of data requires the creation of a model of the BOLD signal over the entire experiment by identifying images that coincide with EEG events of interest, such as IED.10 This model is then used to find voxels with BOLD signal time courses using correlation. A map of the IED-correlated BOLD signal change is then created across the brain (for examples, see Figures 6-1 and 6-2). The identification of the events of interest is usually made by human observers and suffers from the well-documented limitations of this approach.11 In addition to the limits of an observer-derived EEG model per se, not all patients will have IEDs in the scanner, thus presenting a further difficulty. In the two largest case series of continuously recorded EEG-fMRI in focal epilepsy,12,13 around 50% of patients had IEDs during scanning. Approximately 60% of these had a BOLD signal change recorded in the vicinity of the electroclinical localization of seizure onset (known as concordant BOLD signal change; see Figure 6-1 for illustration). This figure represents the “yield” of EEG-fMRI in a given study. It is improved by modeling runs of IEDs rather than single events in addition to improvement in the EEG model by adequate spike detection and classification by the observer.11,13,14 The patterns of IED-related BOLD signal changes can be complex, with clusters commonly observed in close proximity to or overlapping the presumed seizure onset zone and remote from the epileptogenic zone and irritative zone regions of BOLD decrease most often observed at remote sites. The interpretation of these remote changes remains one of the active areas in EEG-fMRI research.
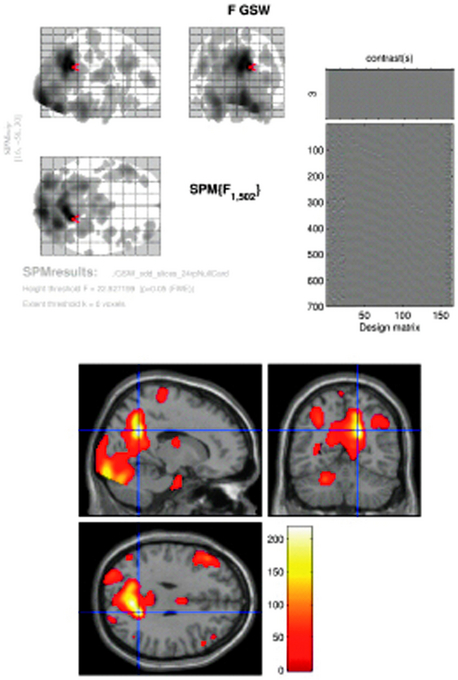
Figure 6–2 SPM{F} from a patient with frequent GSW discharges. Top left: BOLD response correlated with GSW in glass brain display (the red arrow marks the global maxima). Top right: design matrix: first column shows the effect of interest (GSW) convolved with canonical HRF; subsequent columns represent motion and cardiac regressors. At the bottom: BOLD response overlaid onto normalized T1-weighted MRI in MNI space (p < 0.05 corrected). Note bilateral thalamic and right cingulate gyrus activation, right precuneus (global maxima showed by cross hair), bilateral middle frontal gyrus, right occipital lobe (lingual gyrus) deactivation (localization confirmed using Talairach coordinates in Talairach Daemon, http://ric.uthscsa.edu/project/talairachdaemon.html).
Image courtesy of Dr. A. E. Vaudano.
Deviations from the normal time course, however, have been observed in epilepsy. Although the BOLD response, both positive and negative (to external stimuli), has been studied extensively and is well documented in reference to neuronal activity in healthy subjects, some have suggested that this relationship may be altered in the case of interictal epileptiform discharges.16–18 A formal study investigating this possibility found that the inclusion of noncanonical time courses does not lead to an important increase in yield.12 A more recent study suggests noncanonical HRFs associated with IEDs are often remote from the presumed seizure onset zone,19 but whether this represents artifact, propagation of epileptiform activity (time-locked activity) or another phenomenon is not clear. Others have, however, emphasized the importance of intersubject variability in the HRF and routinely use individualized HRFs to analyze IED-correlated fMRI,16,20 and the issue is still under debate. Early BOLD signal change (time locked to IEDs) has been reported in focal epilepsy,21 and BOLD activation has also been reported in the thalamus prior to the onset of generalized spike and wave discharges.22 It is conceivable that these reflect neuronal activity time-locked but preceding an event observed on the scalp EEG and for example may represent a phenomenon similar to observed preictal BOLD signal change in humans25 and preceding the occurrence of epileptic spikes in an animal model.26 Another possibility is that this is a reflection of abnormal neurovascular coupling.
Furthermore the relationship between blood perfusion and BOLD changes linked to epileptiform discharges in focal (one case) and generalized epilepsies are consistent with preservation of neurovascular coupling in epilepsy.23,24
LOCALIZATION OF BOLD CHANGES IN FOCAL EPILEPSY
As has been discussed earlier, IED-correlated BOLD signal change may be colocalized with the irritative or epileptogenic zones.27 We will now discuss the efforts to validate EEG-fMRI localization by comparing other noninvasive localization methods and the gold standard of intracranial recording.
Some reports, predominantly within larger series, of “concordance” of IED-correlated BOLD response with seizure onset were identified by intracranial recording,3,4,12,13 but the only more systematic study was limited to a five-case series. Here it was found that where EEG-fMRI activations were identified, at least one active electrode was on intracranial recording in the same location. The same series made a comparison with source analysis from standard scalp EEG.28
Comparison of noninvasive EEG source localization and EEG-fMRI activations have been made using both spike-triggered fMRI and continuous acquisition. An initial study observed that dipolar sources were often remote (2 to 6 cm) from EEG-fMRI localization.29 This was corroborated by a separate study that suggested distributed source analysis might be a more effective way of comparing EEG source and BOLD activation.30 A recent systematic study using calculated measurements over the cortex revealed IED-associated BOLD clusters that were highly concordant with distributed sources in most patients’ recordings, but also that other EEG-fMRI sources were present that were not concordant with the distributed sources,31 particularly negative BOLD responses. An initial study observed that multi-dipolar sources were generally in anatomical (lobar level) agreement with EEG-fMRI localization based on BOLD increases, and lesser agreement with BOLD decreases.32
Despite comparative studies made between modalities as described earlier, the role of EEG-fMRI in presurgical evaluation remains undefined. The only attempt to assess its practical benefit suggests a limited role in evaluating the group of patients where surgery was not able to be offered.33 Eight patients from this group had significant IED-correlated BOLD signal increase concordant with presumed seizure focus when studied with EEG-fMRI. Of these, four had a unifocal activation, which narrowed the location of the seizure onset zone and was concordant with intracranial recordings in two. In four patients, multifocal BOLD activation concordant with electroclinical evaluation was observed. Larger studies comparing multimodal invasive and noninvasive investigation in addition to postsurgical outcome are required to complete the picture.
BOLD CHANGES ASSOCIATED WITH SPECIFIC PATHOLOGIES IN FOCAL EPILEPSY
It is known in lesional epilepsy that the irritative zone and epileptogenic zone may extend beyond the anatomical boundary of pathological abnormality, and in addition, both in vitro and animal models suggest abnormal subpopulations of neurons within dysplastic areas.34 EEG-fMRI has therefore been used as a tool to evaluate the hemodynamic response in areas of cortical malformation and other pathologies.35–39
In general, EEG-fMRI studies of malformations of cortical development have shown variability in BOLD response within pathologically abnormal regions with broadly concordant activations reported in both gray matter heterotopia35 and focal cortical dysplasia.38 In both these studies, BOLD decreases were observed remote to the area of pathological abnormality. Other studies of malformations of cortical development (MCD) have supported these findings.37 The frequent IED-related BOLD decreases, particularly in MCD, have been attributed to loss of neuronal inhibition (in the presence of normal neurovascular coupling) in the regions surrounding the abnormality or abnormalities in neurovascular coupling itself. The significance of these deactivations will be discussed in more detail later.
A recent study of IED-related BOLD signal change in cavernomas36 suggested BOLD signal change both within the area of anatomical abnormality and within areas remote to it. Caution is required in interpreting BOLD signal change in these very vascular lesions as T2* sequences used in EEG-fMRI are very sensitive to hemosiderin within the lesions.
EEG-fMRI results in tuberous sclerosis reflect a similar pattern. In a recent study exclusively using pediatric patients, it was found that BOLD activation was variable between tubers and extended beyond the border of tubers as identified on structural images once again supporting the view that EEG-fMRI may be a useful technique in assessing the epileptic network beyond areas of structural abnormality.40
EEG-fMRI AND THE PEDIATRIC EPILEPSIES
The pediatric population has been generally less well studied with EEG-fMRI than adults (although some of the studies mentioned earlier have included several children). The experiments are lengthy and can be difficult to tolerate, particularly because they require subjects to remain still for the duration of the procedure. However, intracranial recording is also difficult in children, and invasive procedures such as this may be less acceptable to the pediatric population, meaning that the need for development of noninvasive techniques for localization of the seizure onset zone is even more pressing.41
Two series have used EEG-fMRI in groups of children with focal epilepsies of mixed etiology illustrating that the experiments are tolerated, and results may also show concordance with the seizure onset zone.42,43 In the most recent of these studies, it was found that deactivations are more common and more widespread than in adults, although a common pattern was not identified in this group.44 Questions remain regarding whether this is a function of age and the developing brain, of the particular epilepsy syndrome under study, or whether it in fact is related to pharmacological sedation, the use of which is necessary in children undergoing EEG-fMRI. Previous studies have suggested that benzodiazepine sedation may affect BOLD response to IEDs.45
Specific syndromes are now being studied in children. Two groups of children with benign epilepsy of childhood with centrotemporal spikes (BECCTS) have undergone EEG-fMRI,32,46 and both studies reported BOLD signal change concordant with the electroclinical localization in addition to the tuberous sclerosis study mentioned earlier. A recent study of children with generalized spike-and-wave activity is discussed later.22
ICTAL STUDIES IN FOCAL EPILEPSY
Clinical seizure onset has been used as an event marker in fMRI studies without the use of EEG,25,47,48 which has revealed concordance of seizure-related fMRI activation with electroclinical localization in some cases.
In the few ictal EEG-fMRI studies in focal epilepsy, activations have been more widespread than those observed interictally, usually extending beyond the seizure onset zone. A large increase in BOLD signal was observed, time-locked to a focal subclinical seizure in electroclinically concordant gray matter structures.49 In a patient with multiple simple partial seizures, BOLD activation was revealed, concordant with the electroclinically determined seizure focus. In addition, widespread deactivation was observed on the contralateral side and in other areas of the brain, and increases in the area of activation with cumulative numbers of seizures were analyzed.50
One benefit of recording seizures has been the opportunity to observe preictal changes in BOLD signal. PET and SPECT data suggest preictal changes in cerebral metabolism, and studies of cerebral blood flow support this.51 An fMRI study without simultaneous EEG in three patients suggested BOLD signal change may occur up to minutes prior to the electroclinical onset of seizures,25 a phenomenon that requires further evaluation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree