Fig. 4.1
When is a seizure status epilepticus? Definitions of status epilepticus are still heterogeneous. The typical, self-limited epileptic seizure clearly lasts less than 5 min (GTCS generalized tonic–clonic seizure, CPS complex partial seizure) (Jenssen et al. 2006). The longer an epileptic seizure lasts, the less likely it terminates spontaneously, but the higher is the mortality rate (DeLorenzo et al. 1999). These findings resulted in an operational definition of status epilepticus proposing that every epileptic seizure lasting more than 5 min is status epilepticus, requiring instant and appropriate anticonvulsant treatment
Once the SE diagnosis has been made, antiepileptic treatment (or maybe better, anticonvulsant treatment, as the agents used act symptomatically) needs to be initiated instantly. First-line treatment was assessed in some randomized controlled trials, favoring intravenous lorazepam or intramuscular midazolam (Treiman et al. 1998; Alldredge et al. 2001; Silbergleit et al. 2012) over other agents (particularly, phenytoin). In practice, it is reasonable to administer a benzodiazepine, a fast-acting drug class; of note, diazepam redistributes relatively quickly away from the brain and therefore may be less suitable than lorazepam, midazolam, or even clonazepam. Which of the second-line anticonvulsants is most effective is planned to be assessed in the near future in a multicenter randomized controlled trial (ESETT, established status epilepticus treatment trial) (Bleck et al. 2013). Though a unifying definition of refractory SE does not exist, most authors agree that failure of an intravenous benzodiazepine (in most cases lorazepam) and a second-line drug, such as (fos)phenytoin, levetiracetam, or valproic acid, to terminate ongoing seizures defines refractoriness (Holtkamp et al. 2005b; Rossetti et al. 2005; Hocker et al. 2013). As opposed to first-line therapy, treatment of refractory SE is not based on high-evidence studies. Moreover, it seems reasonable to tune the further extent of treatment aggressiveness according to the clinical form. Complex partial SE (i.e., focal SE without severe consciousness impairment) itself does not seem to increase mortality rate or neurological/neuropsychological long-term sequelae. Therefore, pharmacological treatment regimens should try to avoid anesthetic anticonvulsants, at least initially. In contrast, generalized convulsive SE and its late clinical form – subtle SE (also called nonconvulsive SE in coma) – are accompanied by extensive neuronal excitotoxicity and induce potentially severe systemic challenges to the physiological homeostasis; thus long-term neurological and neuropsychological consequences represent a consistent risk (Meierkord and Holtkamp 2007). After failure of first- and second-line anticonvulsants, anesthetics such as barbiturates, midazolam, or propofol are highly recommended (Meierkord et al. 2010). Approximately, every fifth patient does not even respond to anesthetics, and seizure activity recurs after tapering. This condition has been termed malignant (Holtkamp et al. 2005a) or superrefractory (Shorvon 2011) SE.
The outcome of refractory SE is poorer than SE responding to the first-line treatment. In-hospital mortality has been reported to be as high as 30–40 %, either including (Sutter et al. 2013) or excluding (Hocker et al. 2013) hypoxic encephalopathy. Furthermore, duration of refractory SE is associated with poor prognosis (Sutter et al. 2013); survivors develop chronic epilepsy in 85 % of cases (Holtkamp et al. 2005b). However, even after several days of refractory SE, neuropsychological outcome may be favorable at least in some patients (Cooper et al. 2009); therefore, one should not stop treatment, especially in younger individuals, in the absence of clear signs of irreversible and severe brain damage.
4.3 EEG in Critically Ill: Terminology and Criteria for Seizures (See Also Chaps. 3 and 5)
Clinicians reading EEGs are often asked to judge if an EEG recorded from a critically ill patient is SE or not. However, the SE diagnosis, as that of epilepsy in general, is majorly a clinical one, and the EEG is only one piece of the puzzle. Most periodic EEG patterns are unspecific and per se do not allow to make the diagnosis of SE. In this regard, the commonly used term “epileptiform” in descriptions of generalized or lateralized periodic discharges may be misleading and bears the risk of misdiagnosing nonepileptic conditions for SE. Therefore, one can appreciate the American Clinical Neurophysiology Society’s undertaking to standardize EEG terminology in critical care – and the concept behind it (Hirsch et al. 2013). One of the main goals was to eliminate terms with clinical connotations, such indeed as the criticized term “epileptiform.” Following the proposed nomenclature, the former well-known term “periodic lateralized epileptiform discharges (PLED)” is proposed to be replaced by the descriptive term “lateralized periodic discharges” (Fig. 4.2a). Analogously, “generalized periodic epileptiform discharges” (GPEDs) are now better labeled “generalized periodic discharges” (Fig. 4.2b). As stated, these patterns are unspecific; they do not necessarily indicate SE, but depending on the clinical context and course, they may do (Meierkord and Holtkamp 2007) (Fig. 4.3).
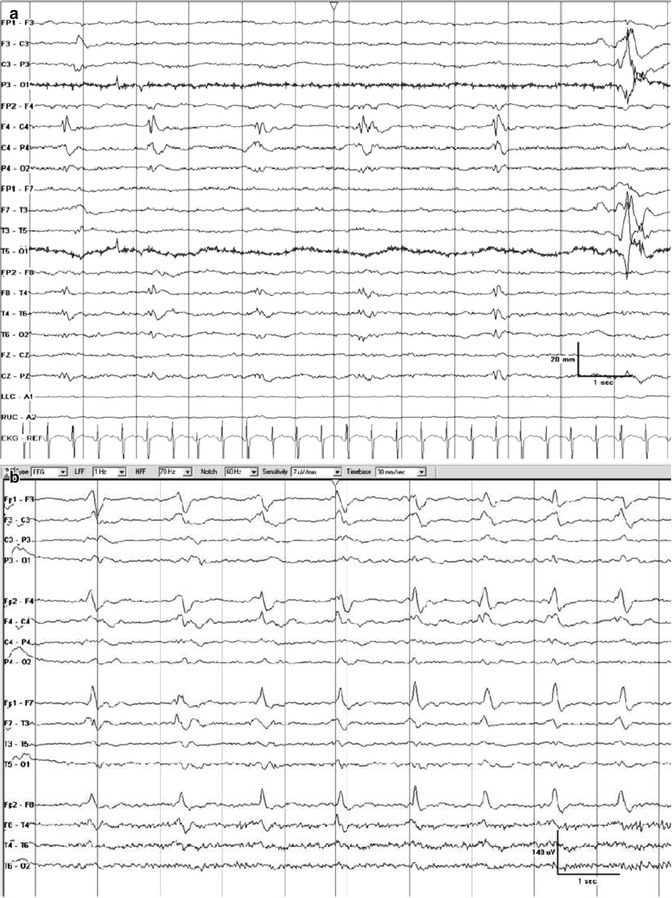
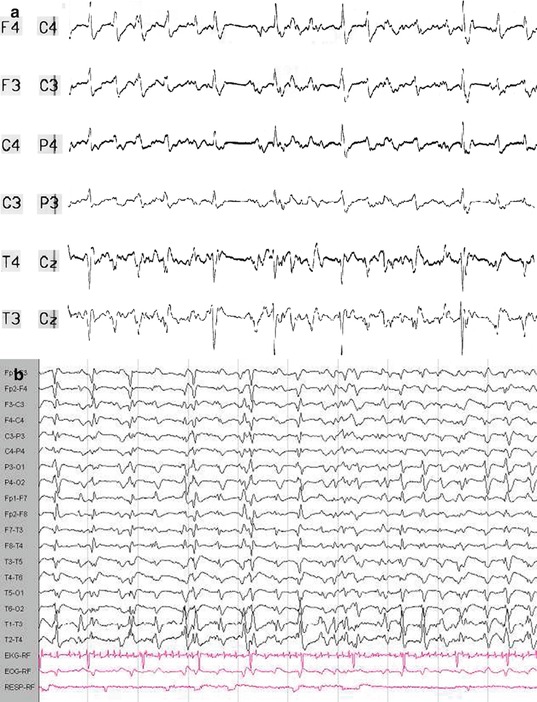

Fig. 4.2
Examples of periodic discharges. (a) Lateralized periodic discharges on the right at a frequency of approximately 0.5 Hz. (b) Generalized periodic discharges with sharp morphology at a frequency of approximately 1 Hz. Information on the clinical condition are not available (Hirsch et al. (2013). With permission from Wolters Kluwer Health)

Fig. 4.3
Generalized periodic discharges. Both EEG traces demonstrate generalized periodic discharges punctuated by flat periods. The correct clinical diagnoses can only be made when the clinical course is considered. (a) This EEG is recorded from a 39-year-old woman who presented with new-onset discrete tonic–clonic generalized seizures that increased in frequency, without regain of consciousness. Etiology was assumed to be encephalitis without identification of a specific pathogen. In retrospect, she may had suffered from immune-mediated status epilepticus, but cerebrospinal fluid or serum specimen was not preserved. In the further course, she had continuous generalized motor seizures. She was treated unsuccessfully with benzodiazepines and phenytoin and subsequently with anesthetic anticonvulsants including propofol and thiopental. Even after tapering of the anesthetics, generalized periodic discharges indicated subtle status epilepticus, thus fulfilling the criteria for malignant (or superrefractory) status epilepticus. Clinically, discrete (“subtle”) spontaneous perioral and extremity myoclonus was observed. Eventually, the patient died from electromechanical dissociation after 8 weeks of critical care treatment. (b) This EEG was recorded from a 58-year-old male patient 5 days after cardiopulmonary resuscitation due to ventricular fibrillation. The patient underwent standard treatment with hypothermia and simultaneous intravenous midazolam. Three days after the end of cooling and sedation, the patient was still comatose; he suffered from stimulus-sensitive myoclonus (occurrence during endotracheal suctioning or touching the patient). In this case, generalized periodic discharges indicate severe hypoxic encephalopathy. The patient was transferred to a rehabilitation clinic; the further course is unknown
Electrographic seizures – either discrete or prolonged – are well defined either by repetitive epileptiform discharges at ≥3 Hz or by epileptiform discharges at 1–3 Hz with clear evolution of the seizure pattern by frequency, location, or waveform (Chong and Hirsch 2005). Figure 4.4 illustrates a discrete electrographic seizure against the background of generalized periodic discharges (Foreman et al. 2012).
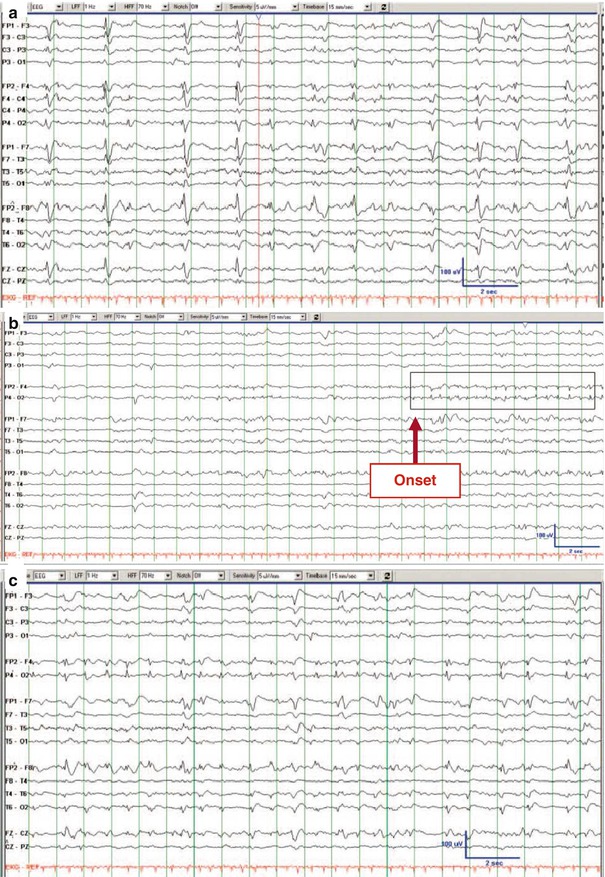
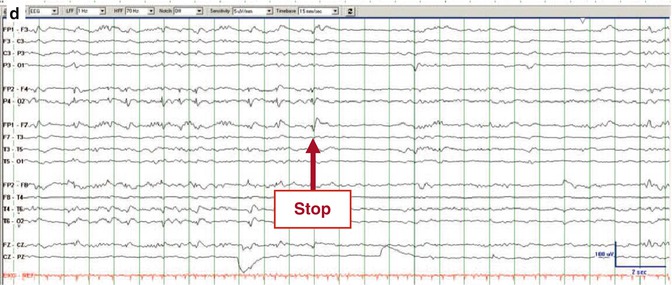


Fig. 4.4
Electrographic seizure. EEG trace recorded from a woman in her 40s, 3 days after liver transplantation complicated by sepsis and renal failure. She was comatose. (a) Her initial continuous EEG monitoring demonstrated frequent generalized periodic discharges, occasionally with triphasic morphology. (b–d) Three consecutive pages of EEG about 2 h later, when she developed focal status epilepticus with right hemisphere onset, maximal in the right frontal parasagittal region (b, red arrow “onset”) that evolved before ending abruptly (d, red arrow “stop”). There was no clinical correlate on video (Foreman et al. (2012). With permission from Wolters Kluwer Health)
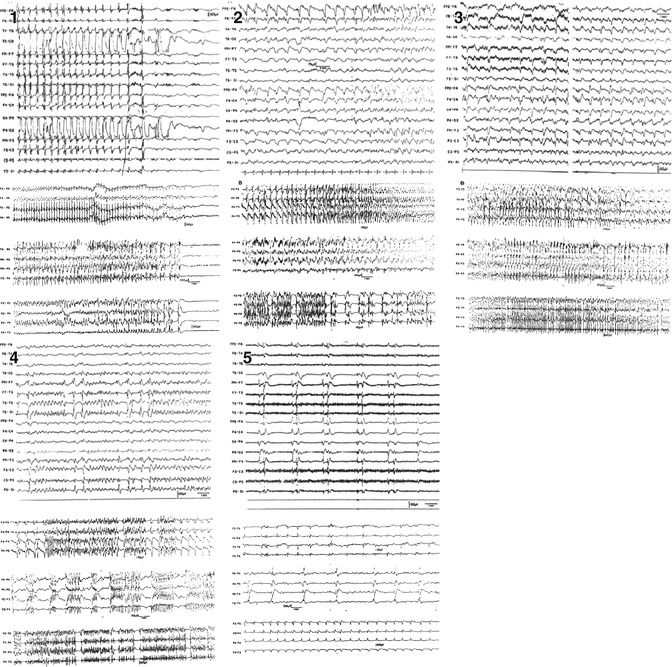
4.4 Temporal Evolution of EEG in Status Epilepticus
The dynamic neurobiological processes underlying SE are well reflected by the evolution of EEG. Treiman was the first to demonstrate that EEG in generalized convulsive SE changes over time following five identifiable and stereotypical patterns (Treiman et al. 1990). At SE onset, (1) discrete seizures were identified that later (2) merge with waxing and waning amplitude and frequency of EEG rhythms. In the further course, the EEG shows (3) continuous ictal activity. With ongoing SE, (4) continuous ictal activity is punctuated by short low-voltage “flat periods,” giving later way to the last pattern, which is characterized by (5) generalized periodic discharges on a “flat background” (Fig. 4.5). These five EEG stages correspond to the extent of generalized motor activity. At onset, (1) single generalized tonic–clonic seizures occur with short intervals that do not allow for full regain of baseline consciousness, defining SE. EEG patterns (2 and 3) correspond to overt generalized convulsive status epilepticus characterized by extensive motor signs. With emergence of EEG flat periods (4 and 5), the intensity of motor activity declines and either disappears completely or presents with only subtle, perioral, or extremity myoclonic twitches.