EEG Traits
Timothy A. Pedley
Introduction
At the most fundamental level, cortical excitability and electrical organization are genetically specified, and it is probable that each identifiable electroencephalographic (EEG) pattern is a heritable trait or made up of a combination of heritable traits. As yet, no specific genetic locus has been identified in humans as controlling any spontaneous brain rhythm, whether normal or abnormal, although this will likely one day be possible. A spontaneous, synchronized EEG pattern has been discovered in the mocha mice mutant that is regulated by a single recessive locus.89 In addition, a defect in a single genetic locus can result in generalized spike-and-wave activity in mutant mice, and a number of independent loci have been identified that give rise to phenotypically similar spike-and-wave patterns, but each of these is associated with different abnormalities of cellular excitability13,88 (see also Chapter 37). In terms of epilepsy, it is now clear that rare epileptic syndromes that aggregate in families result from definable monogenic abnormalities (see Chapters 15 and 18). It is also evident, however, that seizure susceptibility reflects complex alterations in multiple factors governing neuronal excitability. At the present time, recording the EEG is the only readily available method for detecting an abnormal seizure tendency in asymptomatic individuals, or for classifying the physiologic basis of persons with seizures or epilepsy. Electroencephalography has accordingly assumed major importance in the genetic analysis of epilepsy.
Genetic Studies of Electroencephalographic Patterns
Normal Electroencephalographic Activity
The role of genetic factors has interested investigators from the beginning of scientific study of EEG phenomena. A variety of evidence supports substantial genetic influence on patterns of cerebral electrical activity, although results of many studies cannot be accepted uncritically. Davis and Davis20 first pointed out similarities in the EEGs of eight pairs of identical twins, and their observations were corroborated by Loomis et al.75 and by Raney.93 Lennox et al.70,71 studied nonepileptic identical and fraternal twins. Electroencephalograms from monozygotic twins were judged to be identical in 85% of twin pairs, whereas EEGs from nonidentical twins were viewed as different 95% of the time. Later quantitative studies of alpha frequency, voltage and phase relations, and sleep patterns have consistently identified EEGs as concordant from identical but not from nonidentical twins.59,113 Differences between EEG recordings from a pair of monozygotic twins are no greater than the variations that occur spontaneously in sequential recordings from the same individual. Rates of EEG maturation, the appearance and disappearance of age-specific patterns, and, in older subjects, age-related slow activity are all virtually identical in monozygotic twins.101,114,115 Lykken et al.76 studied frequency spectra in twins and found them to be the same in 96% of monozygotic pairs. Butler et al.12 related asymmetries in alpha rhythm to hereditary factors. Vogel et al. have also reported that other patterns aggregate in families, suggesting a genetic basis. These include low-voltage EEG background activity in children,115 variants of the alpha rhythm,116 and some types of beta activity.112,117 The evidence in support of these last examples is not entirely convincing because of the lack of adequate controls for state of alertness, details about drug effects, and the possible influence of anxiety or stress related to the recording methods and circumstances. However, Kubicki67 has also reported that the presence of rhythmic posterior beta activity is under genetic control, and Koshino and Isaki66 demonstrated familial occurrence of the mu rhythm. Although different modes of inheritance have been proposed for some of these patterns, none can yet be accepted as proved. A low-voltage alpha EEG pattern is a genetic trait that has been associated with psychiatric disorders, most notably anxiety disorders and alcohol use.40,41 There is some evidence that this trait is linked to the same region of chromosome 20q as panic disorder. Alpha rhythm traits have not been associated with epilepsy. Ellingson et al.39 studied the occurrence of 14/s and 6/s positive spike bursts in the EEGs of twins and triplets and concluded that this was genetically determined. However, similar rates for 14/s and 6/s positive spike bursts have been reported in the EEGs of unselected and unrelated children.74
Nonspecific Abnormal Electroencephalographic Patterns
Kuhlo et al.68 more fully characterized a rare pattern of 4- to 5-Hz rhythmic activity over the posterior head regions of young adults; this had been described earlier by Vogel et al.116 Once present, this finding persisted in serial EEGs. Kuhlo et al.68 concluded that the pattern was genetically determined, because it occurred identically in two monozygotic twin pairs and in 10% of siblings of affected probands.
Doose et al.27,29 have described “abnormal theta rhythms” in young children, which they correlated with increased susceptibility to febrile seizures and generalized idiopathic epilepsy. The pattern is mainly one of 4- to 6-Hz rhythmic activity, maximal over the parietal areas, and is more common in boys than girls. The finding was strongly age dependent and occurred in about 30% of siblings at ages 3 to 4 years. More recently, Baier and Doose4 have demonstrated an increased risk for generalized epileptiform activity in the EEGs of siblings if probands show abnormal theta rhythms. In the authors’ laboratory, it has sometimes been difficult to distinguish this pattern unequivocally from rhythmic slow activity occurring normally during drowsiness or as part of nonspecifically abnormal generalized slowing of background activity. Another age-dependent finding is 2- to 4-Hz rhythmic activity over the occipital and posterior head regions.46 The genetic features of this, however, are even
less clear. First, rhythmic occipital delta waves occurred as often in siblings of controls as in siblings of delta-positive probands. Second, in younger children (ages 3 to 4 years), occipital delta rhythms were more common in the siblings of controls than in siblings of probands, but the reverse was true in older children (ages 5 to 10 years). Third, delta rhythms did not correlate with epileptiform activity, but their occurrence with generalized epileptiform activity reduced the frequency with which generalized spike-and-wave activity or photoparoxysmal responses were identified in siblings.
less clear. First, rhythmic occipital delta waves occurred as often in siblings of controls as in siblings of delta-positive probands. Second, in younger children (ages 3 to 4 years), occipital delta rhythms were more common in the siblings of controls than in siblings of probands, but the reverse was true in older children (ages 5 to 10 years). Third, delta rhythms did not correlate with epileptiform activity, but their occurrence with generalized epileptiform activity reduced the frequency with which generalized spike-and-wave activity or photoparoxysmal responses were identified in siblings.
Epileptiform Activity
Generalized Spike-and-Wave Activity
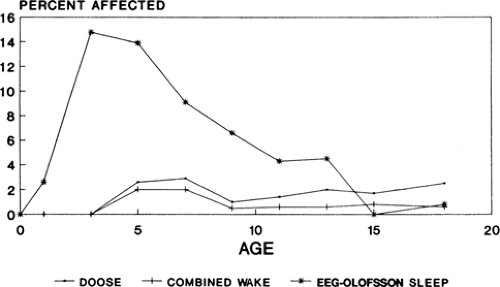
Generalized spike-and-wave (GSW) activity is a genetically determined epileptiform pattern that aggregates in families. Lennox70 first proposed that GSW discharges were the manifestation of a genetic trait, and the now classic studies of the Metrakoses et al.81,82,83,84 unambiguously established the familial occurrence of GSW activity. About 35% of siblings and about 10% of parents of probands with GSW discharges show a similar (but not necessarily identical) epileptiform abnormality in their EEGs.83 Metrakos and Metrakos83 concluded that GSW activity was caused by an autosomal dominant gene with age-dependent penetrance. In the general population, GSW discharges have been reported in the waking EEGs of 0.3% to 1.8% of all children,14,37,38,47 but the finding is age dependent and peak prevalence is 2.8% in children 7 to 8 years old (Fig. 1). If EEGs include sleep, GSW activity can be demonstrated in 15% of normal 3- to 4-year-old Swedish children (7.9% of Swedish children of all ages)38 and in 16.8% of normal 3-year-old Japanese children.106,107 Studies of GSW activity in siblings and offspring are complicated by the presence of epilepsy in most probands. That the two may not be invariably linked is clear from the studies of Metrakos and Metrakos,82,84 who showed that the EEG spike-and-wave trait can be dissociated from clinical seizures. Thus, it is possible that the rates of GSW activity reported in relatives are confounded by the presence of epilepsy, and presumably the type of epilepsy, in the proband. Nonetheless, it is unarguable that a substantial percentage of siblings of children with epilepsy and GSW activity will also exhibit the spike-and-wave trait. Figures range from 7% to 17%,24,47 but as in population studies, rates are age dependent and, perhaps additionally, related to the subtype of idiopathic epilepsy. Thus, 13% of siblings of all probands have GSW activity between the ages of 3 and 6 years.47 In probands with “generalized minor seizures,” the figure increases to 34% in siblings between the ages of 2 and 3 years.25 In children of parents with idiopathic generalized epilepsy, 19% manifest GSW activity in their EEGs,6 and multiple spike-and-wave (“polyspike”) activity occurs in 15% of family members with myoclonic seizures.109 Degen and Degen21 have reported that 72% of siblings of patients with absence epilepsy and nearly 50% of siblings of probands with febrile seizures have EEGs showing generalized 2.5- to 4-Hz spike-and-wave activity, but these data cannot be accepted without further confirmation. The exact mode of inheritance of the GSW EEG trait remains uncertain, and both autosomal dominant and polygenic modes of inheritance have been proposed.
A susceptibility gene at the EJM1 locus on chromosome 6 is involved in the juvenile myoclonic epilepsy phenotype in some families.51 The same gene also seems to influence expression of the EEG abnormality in juvenile myoclonic epilepsy.36,50
GSW discharges most likely involve epileptogenic alterations in thalamocortical circuitry that have been implicated in absence seizures based on studies of spontaneously occurring homozygous mouse mutants11 (Chapter 37), the WAG/Rij rat model of absence epilepsy,80 and supporting clinical data. The three key elements in the circuit are believed to be thalamic relay neurons, thalamic reticular neurons, and cortical pyramidal neurons.15 Abnormalities in this thalamocortical system predispose to absence seizures and characteristic EEG discharges,100 although details remain incomplete. A contributing abnormality appears to be mutations in one of the genes (CACNA1H) that encode the T-type calcium channels that control phasic activation of cortical pyramidal neurons.16,65 Physiologic studies of several of the mutations have demonstrated functional changes in channel behavior that would favor epileptogenic firing patterns.65 Point mutations in the CACNA1A gene, which encodes a Cav2.1 P/Q-type calcium channel, have been implicated in a family in which absence epilepsy is inherited through several generations in an autosomal dominant pattern.57 Several affected family members also have cerebellar ataxia. In all individuals with both ataxia and absence seizures, there was a point mutation in the CACNA1A gene, which encodes the main subunit of Cav2.1 channels. In one asymptomatic family member with typical 3-Hz spike-and-wave discharges, however, the mutation was absent. γ-Aminobutyric acid (GABA)B mechanisms have also been implicated in absence seizures and generalized spike-and-wave discharges.12 Thus, while
thalamocortical circuits, calcium channel mutations, and GABAergic inhibition all seem to be involved in the absence epilepsy phenotype—and probably other idiopathic generalized epilepsy phenotypes as well—details of how individual components of these phenotypes, including differences in the pattern of generalized spike-and-wave activity, remain unclear. Recent studies of genetic absence rats from Strasbourg (GAERS) using genomewide scans indicate polygenic control of specific spike-and-wave discharge variables.96
thalamocortical circuits, calcium channel mutations, and GABAergic inhibition all seem to be involved in the absence epilepsy phenotype—and probably other idiopathic generalized epilepsy phenotypes as well—details of how individual components of these phenotypes, including differences in the pattern of generalized spike-and-wave activity, remain unclear. Recent studies of genetic absence rats from Strasbourg (GAERS) using genomewide scans indicate polygenic control of specific spike-and-wave discharge variables.96
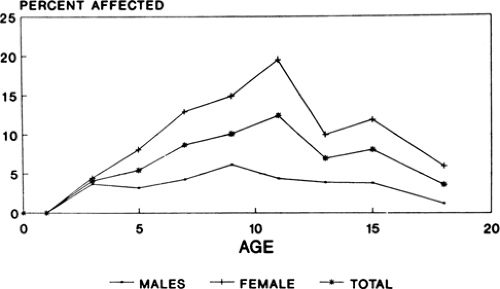 FIGURE 2. Prevalence of photoparoxysmal responses in normal children. (Graphs kindly prepared by Dr. W. A. Hauser and derived from combined data of Doose H, Gerken H. On the genetics of EEG anomalies in childhood IV. Photoconvulsive reaction. Neuropädiatrie. 1973;4:162–171; and Eeg-Olofsson O. The development of the electroencephalogram in normal children and adolescents from the age of 16 through 21 years. Neuropädiatrie. 1971;3:11–45. [Figs. 7 and 8].) |
Photoparoxysmal Responses
Photosensitivity—that is, the development of generalized bursts of irregular spike, multiple spike, and spike-and-wave activity in response to intermittent unpatterned light stimulation—is a familial trait, as first pointed out by Nekhorocheff86 and then studied more completely by Daly and Bickford,17 Davidson and Watson,19 Watson and Davidson,120 Daly et al.,18 Schaper,99 and Watson and Marcus.121 Despite differences in definitions of photosensitivity, criteria for terming a response photoparoxysmal, and recording methods, these early studies found that photoparoxysmal responses occurred in about 20% to 60% of near relatives of probands. Later studies have found somewhat lower rates. Doose and Gerken26 and Doose et al.31,33 compared EEG findings in siblings of children who had photoparoxysmal responses with those of nonepileptic controls. Overall, a photoparoxysmal response could be recorded in about 16% of siblings, but rates were strongly age dependent (Fig. 2). Thus, photoparoxysmal responses to rhythmic light flashes were rare below age 4 but were seen in 32% of siblings ages 11 to 12 years, and abnormal photic responses were more common in girls than in boys. Photoparoxysmal responses occurred in about 5% of control children. Electroencephalographic photosensitivity could be demonstrated in 10% to 20% of siblings of children with epilepsy but without photoparoxysmal responses. The presence of GSW activity in the EEGs of probands did not increase the chance of photoparoxysmal responses occurring in siblings, and photoparoxysmal responses without other EEG abnormalities did not increase seizure risk in siblings. Thus, GSW activity and photoparoxysmal responses appear to be genetically independent phenomena. More recently, Waltz and Stephani118 also studied photoparoxysmal responses in the siblings of patients with epilepsy. The occurrence of photoparoxysmal responses was age dependent with maximum rates seen between 5 and 15 years of age. If one parent was photosensitive, about 39% of all siblings were also photosensitive. However, in siblings between 5 and 15 years of age, the rate was 50%. When a proband was photosensitive but neither parent was, 14% of siblings demonstrated photosensitivity. Sisters were somewhat more likely to be photosensitive than their brothers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree