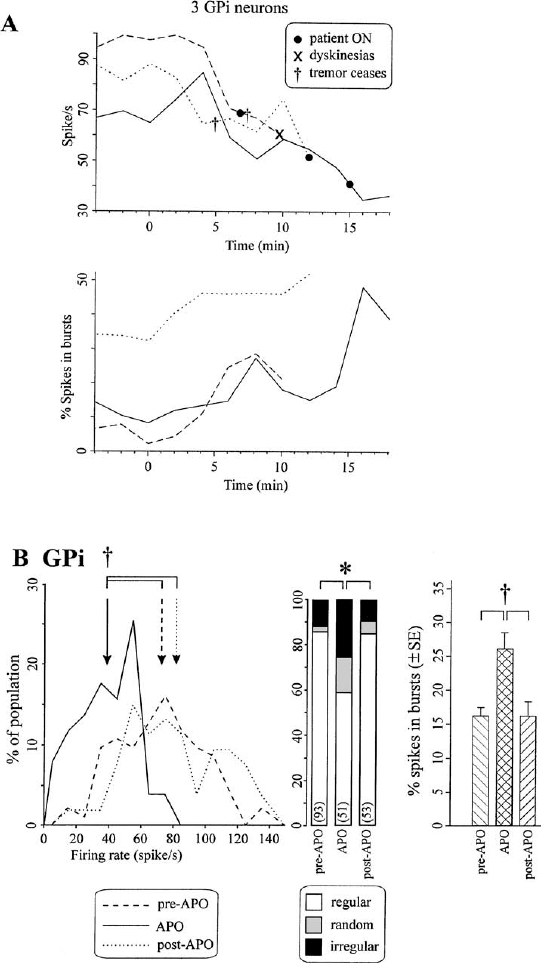
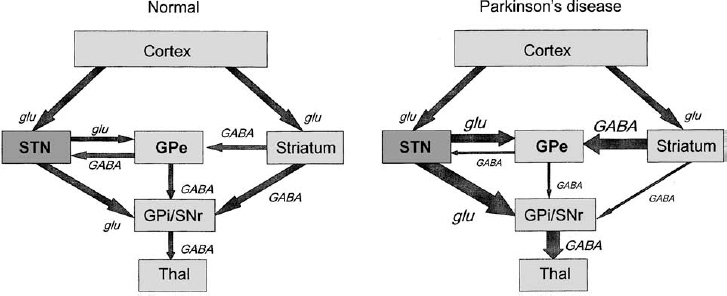
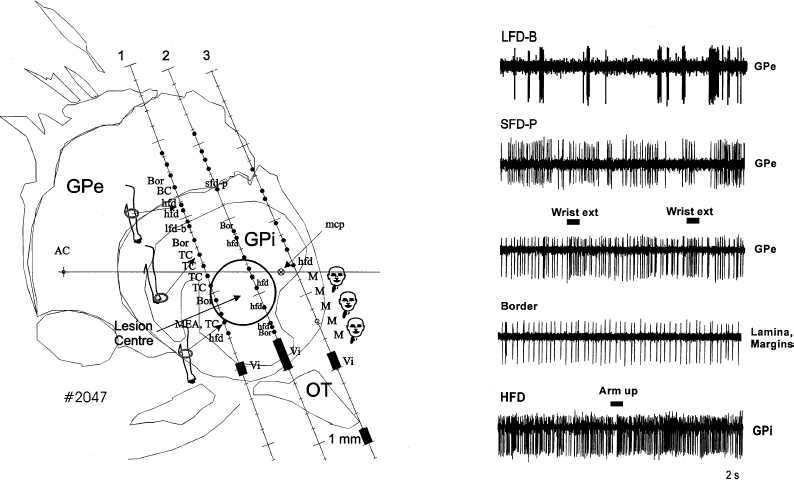
8 Microelectrode recording is used in functional stereotactic surgery to monitor sequentially the neuronal activity of individual cells in the brain, thereby providing a physiology-based map in the vicinity of the targeted structure. Despite advances in medical imaging technology there remains a strong clinical indication for the use of microelectrode recordings, which is largely due to the unpredictable nature and degree of anatomical distortion inherent in magnetic resonance images of the brain. Other sources of error in the procedure are nonorthogonal frame placement, the possibility of brain shift following CSF loss, and other errors that may cancel or compound, leading to variable targeting accuracy.1 For this reason, many centers use a combined image- and microelectrode-based approach to target subcortical structures, which requires more resources and time but provides an added level of assurance. Additionally, microelectrode recording can provide a unique insight into the pathophysiology of the brain of awake individuals with movement disorders. The technique has not become obsolete after many decades of use, but continues to provide a high degree of spatial and temporal resolution of single and multiunit firing behavior with negligible trauma to the neuropil. Indeed, the technique continues to evolve, with the introduction of arrays of electrodes that give further insight into important network-based properties of neuronal aggregates. With the increased use of stereotactic neurosurgery in the post-levodopa era to compensate for the basal ganglia dysfunctions of Parkinson’s disease and other movement disorders, we are in a unique position to gain further insight into the firing rates and properties of human BG neurons in the disease state and their complex responses to normal movements. This chapter will focus on microelectrode-based research findings during stereotactic pallidal procedures largely in PD patients (pallidotomy and pallidal DBS), but also in dystonic patients. The globus pallidus or pallidum is comprised of the external (GPe) (lateral) and internal (GPi) (medial) segments and forms part of the telencephalic BG that includes the striatum (caudate and putamen) lying dorsal and lateral to GPe. Anteriorly, the head of the caudate is contiguous with the more ventral and lateral putamen, but the internal capsule divides these two structures in the coronal planes that contain GPe and GPi, and the caudate nucleus extends posteriorly to form a tail-like structure. The two segments of the globus pallidus together with the putamen are often referred to as the lentiform nucleus. Both segments of the globus pallidus receive major input from the medium spiny neurons of the striatum and are predominantly comprised of GABAergic neurons, but their anatomical connections and functional roles differ.2 A model of normal BG function was proposed based on neuroanatomical and neurophysiological data.3,4 In this scheme, the input to the BG comes mainly from the striatum, which itself receives widespread input from almost all areas of the cortex. The output of the BG derives from the GPi. More recent electrophysiological studies in monkeys5 and rats6 have shown that the subthalamic nucleus provides an important fast excitatory input to both the GPe and the GPi. There are two main projections from the striatum (input) to the GPi (output) that are considered to form the direct pathway from BG input to output (Fig. 8–1). Projections from the striatum to the GPe comprise the first step of the indirect pathway, completed by the GPe projections first to the STN, then to GPi and the substantia nigra pars reticulata (SNr). The GPi/SNr output projects to other motor nuclei such as the ventral anterior thalamus and the superior colliculus, and to brainstem areas such as the pedunculopontine nucleus. Connections with the motor thalamus bring the BG output back to the premotor cortical areas involved in motor preparation and organization, to complete a cortico–basal ganglia loop (not shown in Fig. 8–1). However, other work (see review by Parent and Hazrati2) suggests that the evidence supporting the indirect pathway is weak because the dorsal portion of the STN, which receives GPe input, sends reciprocal excitatory connections back to the GPe instead of to the GPi. Additionally, pallidothalamic projections enable output from the GPe to go directly to the reticular thalamus, thereby avoiding the GPi/SNr entirely. Others have noted more shortcomings of the model, such as the omission of significant pathways, notably the large input to the striatum from the intralaminar thalamic nuclei as well as the SNr.7 FIGURE 8–1 Simplified scheme of a model of basal ganglia function in normal and Parkinson’s disease showing subthalamic nucleus input to basal ganglia via the cortex. Individual nuclei are shown as boxes. The major projections are depicted as arrows, with the major classical neurotransmitter indicated for each pathway. Note that the STN is considered an important input structure for the basal ganglia rather than an intermediary in the indirect pathway. GABA, gamma-aminobutyric acid; glu, glutamate; GPe, globus pallidus externus; GPi, globus pallidus internus; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; Thal, motor thalamus. (Modified with permission from Nambu A, Tokuno H, Hamada I, et al. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300.) Two major types of neurons have been described in GPe. The first type of neuron demonstrates slow-frequency discharges with pauses (SFD-P) and firing rates of ~40 to 60 Hz, with pauses in the range of 300 to 500 msec. The second type of GPe neuron demonstrates low-frequency discharges with bursts (LFD-B) and a firing rate of ~20 Hz, with grouped discharges occurring at irregular intervals and with intraburst firing frequencies at 300 to 500 Hz. In GPi, neurons have been identified with discharge rates in the range of 70 to 120 Hz, termed high-frequency discharge (HFD) neurons. These HFD neurons have fewer pauses and fire with an irregular pattern. Tremor cells have also been found in GPe and GPi, with oscillations in firing frequency that occur at the same rate as the patient’s tremor (see below). The canonical model of basal ganglia function in hypoactive movement disorders such as Parkinson’s disease predicts that the tonic ongoing firing rates of GPe neurons should be decreased, and that of GPi should be increased compared with the normal condition (see right panel of Fig. 8–1). According to the model, the normal role of the dopamine released in the striatum is to maintain activity in neurons that predominantly express D1 receptors and to inhibit activity in neurons expressing mainly the D2 type of receptor. This control of neuronal activity is accomplished by receptor linkage to different second messenger systems that increase or decrease adenylate cyclase. Loss of striatal dopamine leads to decreased stimulation of D1-mediated activity in the direct pathway to GPi and increased stimulation of D2 receptors in the indirect pathway to GPe. Because both of these pathways are GABAergic, the consequence of dopamine loss is a reduction in the direct inhibition, or a net disinhibition of GPi neurons, and an increased inhibition of GPe neurons. The decrease in activity of GPe exacerbates the hyperactivity of GPi because GPe normally has a tonic inhibitory effect on the STN, which in turn has an excitatory glutamatergic influence on GPi, further promoting the hyperactivity of GPi. The proposed net effect is a hyperactive GABAergic outflow from the pallidum to thalamocortical loops involved in the planning and execution of movements. This inhibition of motor control centers is thought to lead to the bradykinesia and akinesia that characterize parkinsonian syndromes, and possibly leads to rigidity and tremor, as well. However, the precise mechanisms of motor symptom manifestation have not been elucidated. Rigidity may arise in part due to elevated BG output to brainstem motor areas,8,9 and tremor at rest is more likely to be a consequence of increased synchrony among neurons of the basal ganglia.10–12 Recent work has also emphasized the importance of the STN as an input structure for the basal ganglia5 and suggested that the GPe might serve as an output structure via the thalamic reticular formation.2 This model of BG function has proved useful in terms of hypothesis generation and testing, and our group has examined several aspects of the dopaminergic modulation hypothesis on GPi and STN firing rates and patterns.13 The first major prediction of the BG model shown in Figure 8–1 is that overall firing rates in GPi should be elevated in PD patients who are off medications (OFF state) compared with what would be expected in normal individuals or in asymptomatic conditions after the administration of dopamine agonists (ON state). The MPTP monkey model of PD provided evidence to support the hyperactivity of neurons in the BG output structures, specifically the somatomotor output from GPi. Although initial studies documented elevated firing rates in GPi neurons in MPTP monkeys,14,15 some discrepancy exists because this was not a consistent feature noted in the study of Bergman et al,16 although the firing rate of tremor cells as a subgroup did appear to be elevated. In this data analysis, the GPi was considered as a single entity and not segregated into dorsal and ventral aspects for firing rate comparisons. An electrophysiological study from our group in some of the earliest of the PD patients undergoing pallidotomy17 indicated that the ventral portion of the internal segment of GPi had overall mean firing rates that were higher than either the more dorsal GPi or the GPe (Fig. 8–2). At the time of that study the division into these groups was made based on examination of firing rate profiles (rates plotted over depth of neurons in the trajectory), and not on the basis of prior knowledge of the distribution of afferent input or anatomical subdivision of the nucleus. The raw data appeared to indicate rate differences between the dorsal and ventral portions of GPi that suggested these regions should be treated as separate groups for analysis. Indeed, the ventrolateral portion of the GPi is thought to represent the somatomotor region, and other evidence indicates that the dopamine deficit in the striatum in PD is not uniform but is most extensive in the putamen. However, 40% of dopamine still remained in most regions of the caudate,18 providing a rationale for differential firing rates in the two regions of GPi that receive input from motor and nonmotor portions of the striatum. Consistent with these findings of functional heterogeneity within GPi, effective pallidotomy for the treatment of parkinsonian symptoms targets the ventral and lateral portion of the GPi.19 Thus, it is not surprising that analyzing dorsal and ventral aspects of the GPi separately discovered only the increase in firing rate from ~65 Hz to 85 Hz. Indeed, an overall analysis of both segments found no difference in firing rates, and the classic papers by Bergman et al16 and Wichmann et al,20,21 did not examine the motor and nonmotor portions of the GPi. It is likely that large numbers of neurons are required to detect this difference in firing rate, given the range of neuronal activity within the GPi. Other models have been developed that suggest that the normal role of the basal ganglia is to focus, scale, and release desired movements by selection of neurons controlling certain muscle groups, whereas neurons controlling postural muscle groups, for example, are held under tonic control.22,23 This may serve to stabilize the kinematics of the body, so as to maintain balance and postural control during reaching, for example. In PD, this function is disordered by changes in striatal dopamine, and there is a deficit in motor control due to impaired surround-inhibitory mechanisms in the BG. One recent study did not demonstrate changes in firing rate following MPTP but noted changes primarily in receptor field properties and increases in inhibitory responses to passive manipulation.24 Although it is possible that changes in firing rate are too small to be significant to PD pathophysiology, recent network modeling of neuronal systems indicates that small changes in the firing rate of relatively large populations of noisy (or irregularly firing) neurons can lead to large firing rate changes (increases or decreases) in secondary or downstream coincidence detector neurons that generate clear binary signals (yes/no or go/no go).27 Recent anatomical tracing studies indicate that the organization of basal ganglia structures appears to be more complex than is usually portrayed in the simpler models of BG function, with a higher degree of convergent and divergent connectivity.25 This tends to support network-based models of BG function where individual nuclei may perform dimensional reduction26 or coincidence detection,27,28 rather than integrating firing rates or patterns of spike trains. The model of BG function shown in Figure 8–1 can also predict the effects of apomorphine not only to ameliorate PD symptoms but also to predict dyskinesia. Whereas PD symptoms are due to excessive output from basal ganglia, abnormal involuntary movements are proposed to occur when neuronal activity in the BG output nuclei (GPi/SNr) is low and the thalamocortical movement–generating circuits are disinhibited. Other specifics of the theory of dyskinesia suggest that the D1-direct striatopallidal pathway is overactive and that drugs with additional D1 agonist properties may be more likely to induce dyskinesia.29 The characteristic pathology of dopaminergic cell loss in the substantia nigra of PD patients leads to a striatal deficit of dopamine that can be pharmacologically reversed by exogenous administration of L-dopa/carbidopa, which is converted to dopamine in the parkinsonian brain. The nonselective dopamine receptor D1–D5) agonist, apomorphine, was used in our studies and those of several other groups30 to monitor the activity of BG output neurons as parkinsonian symptoms reversed and to determine whether a decrease in activity was seen. Earlier studies in MPTP-treated monkeys14,31 indicated that almost all GPi cells were silenced by apomorphine doses and that recovery did occur after ~40 minutes. Studies by Boraud et al24 also examined selective dopamine D1 and D2 receptor agonists and found similar inhibitory effects on GPi neuronsresulted, andeven theselective D1 agonists induced dyskinesia. However, it should be noted that the range of doses of apomorphine in these animal studies (20–200 μg/kg)31 was much higher than those employed in the clinical studies from our group (typically 30–60 μg/kg)13 and others.32,33 As mentioned above, patients received apomorphine doses that were not intended to produce dyskinesia but only to reverse PD symptoms. Despite the attempts to optimize the dose prior to surgery, the variable time and kinetics of dopamine washout led to some patients developing mild dyskinesias during intraoperative recording from the pallidum. The strategy adopted for these studies was intended to maximize the information available from these sessions. Stable single units were selected on the basis of a good signal-to-noise ratio and were well isolated from other spikes that might interfere if there were movements of the electrode tip. The unit was followed until either the firing rate of the cell was significantly reduced, as determined by on-line firing rate histograms, or the patient reported feeling the beneficial effects of the apomorphine, indicating the ON state. The electrode trajectory was then continued, and more cells were sequentially sampled for the remainder of the case as the effects of the drug wore off. Firing rates were calculated, and a burst index was determined based on the temporal dispersion of intervals in the spike train. Previous studies used a burst index based on the interspike interval time histogram (ISITH), which is calculated by finding the mean interspike interval (calculated from the reciprocal of the firing rate) and dividing by the most common or modal ISI. In this method, a regular firing cell such as a border cell, has a Gaussian-like or normally distributed ISITH, the mean ISI is very similar to the modal ISI, and the ratio, or burst index, is around 1. A bursting cell such as that found in the thalamus, has a bimodal ISITH, with most of the spikes occurring in short ISIs of the burst, but the overall firing rate is low when integrated over the total sampled time, and the resultant mean ISI is a high value. In these cases, the ratio of mean to modal ISI is a value around 10. GPi cells, which normally have an irregular firing pattern, usually have a ratio of mean to modal ISI of 2 to 3. More recent analysis33a used a simplified version of the Kaneoke and Vitek33b method based on the Poisson distribution to characterize the discharge properties as regular, random, and irregular. The majority of GPi neurons come to be classified as regular in this scheme, even though their firing pattern in most cases would not be described as such. However, as a means of quantification for comparison with the drug groupings, this latter technique has been useful. To examine further the workings of the model and to confirm the hyperactivity of GPi, apomorphine was administered subcutaneously (2.5–8 mg/patient) to reverse the PD symptoms and to observe the effects on cell firing. In patients whose medication was withheld on the night before surgery (practically defined OFF state), three neurons were continuously monitored for at least 10 minutes following APO, until a significant change in firing rate occurred or the patient felt ON, after which time more units were sampled to look at the population. Apomorphine administration inhibited the spontaneous ongoing firing rate of GPi neurons, consistent with the model shown in Figure 8–1 and with previous studies13,30(Fig. 8–3). The average degree of inhibition at 10 minutes where all three cells were still being monitored was ~30% of the baseline firing rate. All patients reported being ON after the medication, two had cessation of tremor 5 minutes after apomorphine, and one developed dyskinesias ~4 minutes after cessation of recording. The firing pattern of these cells was altered to a state where more spikes were present in bursts, as shown in the lower panel of Figure 8–3A. In the population study (Fig. 8–3B), the frequency distribution of firing rates was shifted from values of ~80 Hz to 40 Hz values and returned to 90 Hz after the effects of apomorphine wore off, further evidence that the effect of apomorphine is to decrease the overall firing rate of BG output neurons. There was no significant rebound to a higher firing rate in GPi neurons after the effects of apomorphine wore off, as noted in our previous study,13 but only a slight shift in that direction (Fig. 8–3B, left). The firing pattern was also altered, with a significantly greater percentage of cells having a random or irregular firing pattern (chisquare test, p <. 01) (Fig. 8–3B, middle), and the percentage of spikes occurring in bursts as determined from the Poisson surprise method went from ~16% preadministration of apomorphine to 26% and returned to the predrug level after 60 minutes (Fig. 8–3B, right). Interestingly, there was no difference in the firing rate in those patients who developed dyskinesias during apomorphine administration and those who did not, suggesting that the involuntary movements themselves were not caused solely by low firing rates of GPi neurons. This observation is in some respects consistent with a study of dopamine agonists administered to MPTP monkeys that suggested changes in the firing pattern of GPi neurons correlated with the onset of dyskinesias.34
Electrophysiological Findings in GPe and GPi
WILLIAM D. HUTCHISON
General Anatomy and Cell Types of GPe and GPi
GPe and GPi in Models of BG Function
Studies on Firing rates, Patterns, and Synchrony in GPe and GPi following Apomorphine
Basal Ganglia Models and Effects of Apomorphine in Nonhuman Primates
GPi Firing Rates and Patterns with Apomorphine in PD Patients
METHODOLOGY
Results of Single Units Followed in GP
Neupsy Key
Fastest Neupsy Insight Engine