Fig. 18.1
(a) Representative figure demonstrating an intraoperative stereotactic system that displays the standard orthogonal views based on the preoperative MRI scan. A trajectory view or the intraoperative endoscopic high-definition video feed can be displayed also to cross correlate the anatomy. (b, c) Intraoperative view in a patient that received intrathecal fluorescein prior to the start of surgery. The green-tinged cerebrospinal fluid demonstrates the location of the arachnoid defect and the need for a watertight closure. The fluorescein-stained cerebrospinal fluid can be visualized with white light (a) or with a blue light filter with a blocking filter
18.2 Surgical Techniques
18.2.1 Use of Intrathecal Fluorescein
The presence and site of a CSF leak can be verified using a combination of diagnostic methods including β-2 transferrin testing, nuclear pledget scan, rigid nasal endoscopy, CT imaging, MR imaging, cisternography, and intraoperative intrathecal fluorescein. The intrathecal administration of fluorescein to stain CSF has been described for over 40 years and is an important adjunct to endoscopic repair of CSF rhinorrhea [33, 34, 37]. Intrathecal fluorescein is helpful in localizing the CSF leak, determine the extent of the defect, and ensure a watertight closure. It is equally of great value in facilitating closure of large defects following endoscopic skull base surgery. The green color is easily distinguished from the surrounding blood and secretions in the operative field that may otherwise obscure the naturally clear, translucent CSF. Although the use of intrathecal fluorescein is generally considered safe [25], older reports have described complications, including seizures, radicular symptoms, and transient paraparesis or hemiparesis [22, 25, 26, 28]. The dosages of fluorescein varied in these reports, and patients were not always premedicated to avoid a reaction to the drug. With use of low-dose intrathecal fluorescein with premedication, these complications have been avoided [24, 37, 38]. We have used low-dose intrathecal fluorescein in over 300 endoscopic endonasal procedures without any adverse-effect incident.
Patients are generally premedicated with 50 mg of diphenhydramine and 10 mg of dexamethasone following induction of general anesthesia. Prior to the procedure, a lumbar puncture is performed to enter the intrathecal space and withdraw 10 mL of CSF. This is mixed with 25 mg of fluorescein (0.25 mL of injectable 10 % solution, Akorn Inc., Buffalo Grove, IL) and is slowly reinjected into the intrathecal space. In patients undergoing planned lumbar drainage, the CSF is obtained following drain placement, and the lumbar drain is clamped following fluorescein injection. The intrathecal injection stains the CSF that is already in circulation.
Endoscopic visualization of the fluorescein-stained CSF is performed throughout the surgery with either a white light or a blue light filter (465–495 nm) with a blocking filter (515–555 nm). The operative field is inspected at the time of the surgical approach to confirm the location and volume of any CSF leak in patients with suspected preoperative CSF leak or encephalocele. In patients undergoing endoscopic tumor resection, the operative field is inspected for CSF leak following complete tumor removal and following reconstruction [24, 37, 38].
18.2.2 Endoscopic Repair of Skull Base Defects
The principles of endoscopic reconstruction of skull base defects are similar to those of traditional open craniotomy approaches, that is, to completely separate the cranial cavity from the sinonasal cavity and eliminate dead space. This closure, especially for large defects with high flow of CSF output, relies on a multilayered reconstruction to reestablish tissue barriers as is true in traditional open craniotomy techniques. The closure is achieved using a variety of techniques based on the severity of the intraoperative leak as well as the location and size of the cranial base defect [29, 39]. The first step of the surgery is to adequately expose the entire defect. The meningocele or encephalocele is resected, and the bony edges of the defect are defined circumferentially. The herniated dura should be resected or reduced into the intracranial cavity. Brain that has herniated into the nasal cavity is rarely functional and considered a potential source of intracranial infection if not resected. However, in certain rare instances of very large encephaloceles, the preoperative imaging must be carefully scrutinized to ensure that important vascular structures, such as the anterior cerebral arteries, have not herniated through the large defect. Another important step is to remove the mucosa surrounding the defect to allow the graft to adhere firmly to the skull base. These maneuvers will often worsen the CSF leak but is critical to obtain an eventual watertight seal and to define the bony defect.
Small isolated CSF leak/encephaloceles with a small bony defect can be closed with a single layer of autologous fat or fascia placed through the defect as an inlay, followed by application of tissue sealant. We also like to use Duraguard (Biovascular, Inc., St. Paul, MN) since it is more rigid than fascia lata. In some situations we have placed Medpor (Porex Surgical, Inc., Newnan, GA) as an inlay to cover the defect with a fascia lata onlay. A final layer of DuraSeal (Covidien, Hazelwood, MO) is helpful to maintain a watertight closure until a fibrotic seal can be obtained. Sellar lesions involving a bony skull base defect without arachnoid violation or intraoperative CSF leak can be closed by packing the cavity with Gelfoam and reconstructing the bony sella with vomer or Medpor graft. Reconstruction is achieved by placing the graft, which has been trimmed to size, as either an underlay or, more commonly, an onlay graft. Sellar reconstruction in the presence of intraoperative CSF leak is achieved by packing the tumor cavity with autologous abdominal fat followed by reconstruction of the bony sella (with vomer or Medpor) and application of tissue sealant [24, 37].
Patients with larger skull base defects after endoscopic endonasal surgery for sellar and anterior skull base tumors (suprasellar, fovea ethmoidalis, cribriform plate) with a high-volume intraoperative CSF leak require multilayered closure [3, 5–10, 14, 16–20, 23]. This can be achieved often with an autologous fat graft in the tumor cavity followed by inlay placement of a fascial layer (harvested autologous fascia lata) and onlay placement of a bony buttress and application of tissue sealant. In cases involving direct communication between the ventricular spaces and the tumor cavity, packing of fat into the tumor cavity is avoided to minimize the risk of iatrogenic hydrocephalus [24, 37].
18.2.3 Gasket Seal Closure
These larger skull base defects usually created after endoscopic endonasal transphenoidal, transethmoidal, transcribriform, transclival, or transpterygoidal approaches to skull base tumors can be supplemented often with a gasket seal closure [24, 37] (Fig. 18.2). Gasket seal closure begins with elimination of any dead space with autologous fat to prevent pooling of CSF over the closure. A gasket seal closure is favored when the defect is >1 cm in diameter and consists of a soft tissue graft (generally fascia lata) centered over the defect with the edges of the graft exceeding the bony defect circumferentially. The radius of the graft must exceed the bone defect by at least 1 cm. If fascia lata is not available, we use bovine pericardium or AlloDerm (LifeCell Corp., Branchburg, NJ). These substances are sufficiently thick to create the gasket seal. Next, a piece of rigid material such as vomer or Medpor (Porex Surgical, Inc., Newnan, GA) is fashioned to be roughly the size of the bony defect and is centered over the soft tissue graft so that there is at least 1 cm of tissue graft extending circumferentially around the rigid graft and gently countersunk into the bony defect, forming a watertight gasket seal around the rigid graft [24]. Bioabsorbable plates and cartilage are not recommended; the former may not last long enough to ensure stable closure, and the latter is not sufficiently rigid. The closure is inspected to make sure there is no leakage of fluorescein-tinged CSF, which would indicate inadequate closure. If CSF is still leaking, either the closure is redone or a lumbar drain is placed. These types of large defects are then further covered with a vascularized nasoseptal flap followed by a final layer of DuraSeal to keep the flap in place.
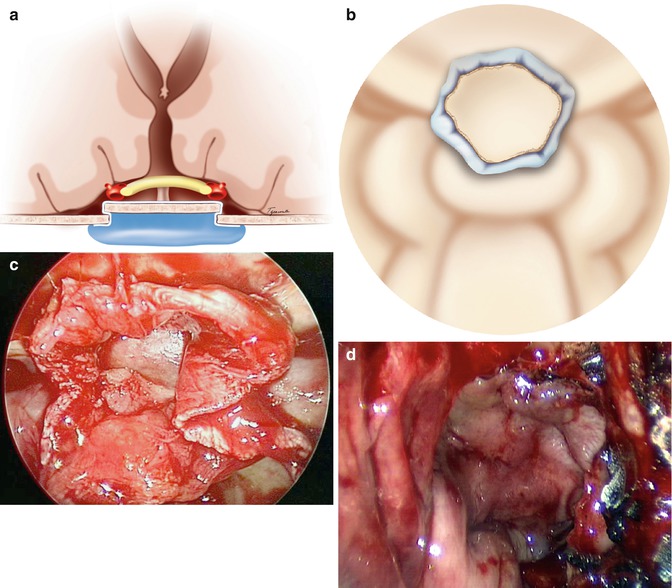

Fig. 18.2
(a) Diagrammatic demonstration with a coronal view of the suprasellar contents and closure of the skull base defect with a gasket seal. The countersunk rigid buttress is cut to the same size as the skull base defect and countersunk with overlying fascia lata for a watertight seal. (b) Diagrammatic demonstration of the intraoperative view of a gasket seal closure similar to (c). (c) Intraoperative image of a gasket seal reconstruction of the skull base. Note the large margin of overhanging fascia lata and the countersunk bone. (d) Intraoperative view demonstrating the nasoseptal flap secured in place
18.2.4 Vascularized Nasoseptal Flap
The use of a pedicled nasoseptal vascular flap of the nasal septum mucoperiosteum based on the nasoseptal artery has become an important adjunct in the endoscopic reconstruction of large skull base defects. The nasoseptal flap is generally harvested at the beginning of the operation, before tumor removal and before the posterior septectomy is performed. The vascular supply of the nasoseptal flap is derived from the posterior septal artery, a terminal branch of the internal maxillary artery [11, 21]. The flap is raised by placing two parallel incisions within the septal mucosa, one along the nasal floor and the other just inferior to the most superior aspect of the septum. These incisions are joined anteriorly to create the flap, and posteriorly these incisions are extended over the rostrum of the sphenoid superiorly and to the choana inferiorly. The flap is elevated anterior to posterior and lateral with a dissector and tucked out of the way of the surgeon into the nasopharynx.
During skull base reconstruction, the nasoseptal flap is incorporated as a final layer of closure in addition to the previously placed gasket seal or bony buttress and inlay or inlay graft. The flap is secured in position with fibrin glue or DuraSeal ensuring that it is not twisted along its pedicle and that the mucosal surface is facing the nasal cavity. It is important that the flap abuts the skull base defect and surrounding bone and mucosa directly without any interposing hemostatic material. Furthermore it is important that the flap is planned large enough to cover all the edges of the skull base defect. After reconstruction and securing of the flap in position, Floseal (Baxter Inc., Vienna, Austria) is routinely placed into the sinonasal cavity, and small folds of Telfa are placed into the anterior nasal cavity and removed on the first postoperative day.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








