Fig. 9.1
Example of a small type II hamartoma suitable for endoscopic treatment. T-2-weighted sagittal (left) and coronal (right) MR images showing a hypothalamic hamartoma with vertical insertion plane and intraventricular location (Used with permission from Barrow Neurological Institute)
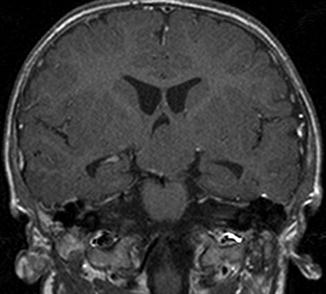
Fig. 9.2
Example of a big type IV hamartoma requiring multiple-step surgery. T-1-weighted coronal MR image showing a giant hamartoma with wide insertion bilaterally and an intraventricular extension
Depending on the anatomical position, the surgeon has to choose the correct approach or a combination [9]. In most cases, the HH is primarily attached to the hypothalamus on one side. Less often, an equal bilateral attachment is present. Apart from the cases presenting only with precocious puberty that can be treated medically, all other patients usually demand a more invasive method [10, 11].
9.3 Management Options
The various treatment options include surgical excision or disconnection through transcallosal interforniceal approach, orbitozygomatic approach or endoscopic approach, gamma knife, stereotactic radiofrequency ablation, stereotactic laser ablation, and deep brain stimulation [12, 13]. Usually the surgery of HH is a multistep surgery [14]. No single approach is the best or is appropriate in all cases [15, 16]. It is important to remember that a HH cannot be distinguished from normal hypothalamus under microsurgical view. Only the abnormal anatomy that it forms allows the surgeon to determine where to stop resection. Hence, assistance from image guidance is of paramount importance, regardless of surgical technique and approach.
The transcallosal, interseptal, interforniceal approach is the preferred approach for large HHs with a significant intraventricular component located superior to the level of the optic tracts [17] (Fig. 9.2). This approach can be used alone to treat large type II lesions. Many type III and IV lesions require a staged approach. If the lesion is entirely medial to the line of sight down the wall of each hypothalamus, then a large type III or IV lesion can be disconnected during one operation. In young patients (<6 years old) and in patients with a small residual cavum septum, the leaves of the septum pellucidum are easily separated. This feature facilitates safe separation of the fornices. As the patient ages, the interforniceal dissection becomes more difficult (used until 12 years of age). It is a long reach to the inferior margin of large type III and IV HHs and often requires working at the tips of the microsurgical instruments with a reduced amount of control. A typical disadvantage of this approach is that the removal of very large lesions carries high risk of severe problems with sodium, but it is useful in moderate lesions in children especially when attached on both sides. Another problem of this method is with adults who have been on anticonvulsants all their lives and they have very thick skulls adherent to sagittal sinus.
The modified orbitozygomatic craniotomy is another valid surgical option because it can maximize working space and light, minimize brain retraction, and achieve as low and lateral of an angle as possible to lesions with bilateral attachment [18, 19]. The key to successful visualization of the inferior hypothalamus, mammillary body, optic tract, and pituitary stalk is achieving a very flat or even upward-looking trajectory. This trajectory is best obtained through a supraorbital craniotomy by extradural drilling of the ridges formed by the orbital part of the anterior skull base. In the case of the orbitozygomatic approach, it is performed by removing the orbital rim and orbital roof. The modified orbitozygomatic approach and a wide sylvian fissure dissection allow the most lateral and upward angle for disconnection of lesions with bilateral attachments. Standard subfrontal dissection and wide splitting of the proximal sylvian fissure are performed.
Endoscopic resection is preferred as the stand-alone surgical treatment for small type II HHs (Fig. 9.1) and as a stage in the treatment of small type III HHs [20, 21]. Stereotactic guidance is required in these cases for three reasons. The entry point is chosen based on using the trajectory views provided by the stereotactic software. The trajectory of resection is determined by tracking the end of the endoscope and again, using the trajectory views to best estimate the course of the HH/normal hypothalamus interface (Fig. 9.3). Endoscopic entry into a small ventricle is often aided by stereotaxy. The entry point is determined by finding the point on the scalp that is intersected by a line drawn from the anterior edge of the side of HH attachment to the hypothalamus and the anterior edge of the contralateral Monro foramen. This is easily done using the trajectory views provided by the stereotactic software. Thus, a right-sided HH will be approached from the patient’s left side (Fig. 9.4). Once the entry point is chosen, a generous burr hole is made, and the dura is coagulated and opened. The pia is also coagulated and opened. We then place a peel-away sheath into the brain along the appropriate trajectory and stop just short of the ventricle. Most patients do not have hydrocephalus, and blindly placing a sheath in a small ventricle is challenging. Once the sheath is placed, we use a stereotactically tracked 30° endoscope and endoscopic visualization to advance the tip of the scope into the ventricle. Once the ventricle is entered, gentle irrigation is infused to induce mild ventriculomegaly, and the sheath is advanced over the endoscope until it is just inside the lateral ventricle. Placement of the sheath in the ventricle relieves the ventriculomegaly and leaves a small space within which to work. The choroid plexus and anatomy of the ventricular venous system guide the endoscope through the Monro foramen and into the third ventricle. Care must be exercised to avoid forcefully impinging on the fornix at the anterior margin of the foramen. While the tip of the endoscope is in the third ventricle, the fornix is not visible. The entry point is chosen as described above because the fornix will not tolerate anterior “windshield wiping” of the endoscope. Gentle posterior “windshield wiping” movements are better tolerated but should be minimized. Once the third ventricle has been accessed, gentle irrigation will separate the walls enough to provide a clear view of the anterior and posterior margins of attachment. The endoscope is secured in place using a robotic manipulator arm. We then obtain microelectrode recordings in the HH for research purposes. A distinct cleft or indentation marks the border of the HH attachment to the hypothalamus (Fig. 9.5). Using this cleft as the starting point, we use the stereotactic trajectory view to determine the angle of the disconnection. The resection begins by using a grasper through the working port of the endoscope (Fig. 9.6). We start at the posterior edge of the HH and work anteriorly. This cycle is repeated until the pial surface on the deep side of the HH is reached. As the disconnection proceeds deeper, the remaining disconnected HH falls away medially from its hypothalamic attachment. We resist the temptation to pull out large chunks of the HH as it is disconnected. Doing so makes the interface swing laterally and increases the difficulty in disconnecting the most inferolateral attachment. Once the HH is disconnected, it is grasped and the endoscope/grasper complex is removed from the ventricle in one piece. If the HH is too large to fit through the foramen, it is morcellized before it is removed. Irrigation with or without coagulation is used to stop any bleeding. A ventriculostomy catheter is typically left in place and removed the next day. One surgical pearl needs to be emphasized: The HH cannot reliably be distinguished from the hypothalamus based on the difference in the color or consistency. Hence, such clues cannot be relied upon to guide resection. It is best to think of the resection as a straight-line disconnection guided by the initial angle determined by stereotaxy and proceed down that line until the deep pia/arachnoid surface is reached or the inferior margin of the lesion as determined by neuronavigation is reached. By not breaching the pial surface, deeper structures such as the optic tract and perforating vessels remain beyond the reach of the instruments. Breaching this surface and damaging the perforating arteries can result in small infarctions of the basal ganglia and internal capsule. Such infarctions are often, but not always, clinically silent. If any part of the HH is positioned superior to the mammillary body, the disconnection must be shallow over the mammillary body to avoid its injury [22]. However, many of these patients are so clinically and socially impaired that clinically significant damage to a mammillary body or fornix would not be evident. For this reason, it is reasonable to be more aggressive in pursuing resection or disconnection and in tolerating damage to relieve epilepsy in severely affected patients.