Fig. 7.1
EasyGO-System (Karl Storz Company, Tuttlingen, Germany). (a) Working trocar with insertion for rod-lens (EasyGO®-system, Karl Storz Company, Tuttlingen, Germany). (b) Rod-lens with light source cable and HD-camera. (c) Tubular working trocar with inserted endoscope. (d) 16:9 wide screen monitor and cold light transmission Endoscopic Spinal Surgery
For spinal endoscopy, a standard endoscopic system consists of three components:
1.
The endoscope which is usually a rod-lens endoscope because of its superior optical quality.
2.
The HD-camera which allows excellent image quality and a 16:9 wide screen video monitor.
3.
A Xenon light source for cold light transmission.
Endoscopic spine surgery was first described in 1983. Since then endoscopic visualization and surgical equipment were continuously further developed and redefined. Currently, endoscopy is applied in cervical, thoracic and lumbar spine surgery. First clinical results were reported on lumbar discectomy procedures by Kambin in the early 1990s [25–27]. In 1997, the first tubular retractor system was introduced to the market by Foley. The idea behind was the application of the microsurgical technique for endoscopic lumbar discectomy (MED) via minimally invasive approach. The spine is accessed via serial dilation of the back muscles. This leaves the supporting musculoligamentous structures intact which are located in the midline [28]. The initially poor image quality of telescopes and an associated flat learning curve of the surgeon deterred many spine surgeons from performing endoscopic spine surgery. Further the lack of depth perception and 3D vision reduced the acceptance of the MED technique. As a consequence of this, a new generation, the so called METRx system, was developed. It provided an increased working space and better illumination of the surgical field. Many surgeons didn’t want to miss minimally invasive surgery and therefore performed procedures by using the operating microscope or loupes instead. Multiple studies have identified that MED provides the same clinical outcome as microdiscectomy with the advantages of less soft tissue trauma and better aesthetical results [29–31]. Tubular systems became popular soon not only because of good clinical results. The indication for the use of tubular retraction systems in lumbar spine is wide and very similar to conventional open procedures [15, 32]. It is not surprising that microendoscopic techniques were applied to treat pathologies which were traditionally operated on in microsurgical technique.
Anterior and Posterior Approaches and Techniques
Since the introduction of the endoscope to spinal surgery, new endoscopic surgical techniques have been constantly developed. If we look at the cervical spine anterior and posterior approaches have to be subclassified. Further if we talk about endoscopic surgery we have to differentiate these techniques into fullendoscopic and endoscopic assisted surgery. The term fullendoscopic refers to surgical techniques on what the visualization of the surgical field is performed by an endoscope at all time of the tissue manipulation only. The term of endoscopic assisted refers to a technique on what the majority of the procedures is performed without the visualization of the endoscope. The endoscope for example is used sequentially during the procedure to insect a part of the surgical field or to manipulate under endoscopic visualization partially.
In the following session the authors will give a brief review on the common fullendoscopic techniques which are applied anteriorly and posteriorly to the cervical spine.
Cervical Microendoscopic Discectomy and Fusion
Anterior Percutaneous Endoscopic Cervical Discectomy
Endoscopic Posterior Cervical Laminoforaminotomy
Microendoscopic Cervical Laminoplasty and Laminectomy
Other techniques like endoscopic assisted transoral odontoidectomy, endoscopic transnasal resection of the odontoid or endoscopic posterior fixation are excluded.
Cervical Microendoscopic Discectomy and Fusion
Anterior cervical discectomy and fusion (ACDF) was first described in the 1950s. Since then it became the standard treatment for degenerative cervical disc disease [33–35]. However, ACDF is associated with certain disadvantages such as donor side morbidity in case of bone graft harvesting at iliac crest, graft dislocation, graft subsidence, nonfusion with consecutive pseudarthrosis, esophageal injury, dysphagia, recurrent laryngeal nerve palsy, etc. [36, 37]. Anterior cervical discectomy without fusion (ACD) offers good clinical results but has a higher risk of postoperative segmental kyphosis and postoperative axial pain [38]. In the past decades a variety of different intervertebral implants have been developed to prevent autologous bone harvesting and donor side morbidity. Further minimally invasive techniques were developed to reduce tissue trauma while approaching the spine. It has been proved that minimally invasive techniques for approaches to spinal pathologies preserve healthy tissue and reduce surgical associated morbidity, shorten operative time, decreased complication rates, reduces hospital length of stay, cause less postoperative use of narcotics, enable faster patient recovery and offer lower costs [26, 39]. Depending on the material, minimally invasive techniques can be limited to certain pathologies and indications. The percutaneous endoscopic cervical discectomy as an alternative to ACD is considered to be indicated in cervical disc herniations which have to be soft and contained or noncontained but without sequestration and contained by the posterior ligament [40, 41]. Cervical microendoscopic discectomy and fusion (CMEDF) is an alternative technique for ACDF that reduces the surgical morbidity of conventional surgery but without limited indications for treatment of cervical degenerative pathologies. This chapter will deliver an impression for the endoscopic technique and equipment that is necessary to perform CMEDF and gives a short review about clinical results.
Indications
The CMEDF approach in indicated in the following cervical pathologies and situations [42–44]:
Central and lateral cervical disc herniations or osteophytes associated with neck injury
Discogenic radiculopathy
Discogenic myelopathy
Spondylotic myelopathy
Discogenic myeloradiculopathy
Axial neck pain, lost cervical lordosis, reduced disc space height
Magnetic resonance image (MRI), computed tomographic (CT) scan or postmyelogram CT-scan that is positive for spinal cord or verve root compressing pathologies consistent with dermatome of clinical symptoms
Failed improvement of symptoms after conservative treatment for 12 weeks
Surgery can be performed from mono- to trisegmental pathologies that involves the levels C3–4, C4–5, C5–6, and C6–7
Contraindications
Compressive pathology located behind the vertebral body (OPLL)
Severe spinal canal stenosis
Surgical Equipment for CMEDF
The following surgical equipment and instruments are necessary to perform CMEDF:
Tubular dilators and tubular retractor system (e.g. METRx, Sofamor Danek, Memphis, USA)
Video digital endoscopy unit with a camera
Different endoscopes (e.g. 0°-optic and 30°-optic)
Ordinary endoscopic spine instruments
5-mm osteotome
Cage (e.g. Polyetheretherketone (PEEK))
Fluoroscopy (C-arm)
Surgical Technique
The CMEDF procedure is performed under general anesthesia with orotracheal intubation. Occasionally the procedure can be performed under local anesthesia in younger patients. Preoperative antibiotics are admitted and dexamethasone might be administered to minimize airway and esophageal edema. The patient is positioned supine with the neck slightly extended. The head might be rigidly affixed via pins in a Mayfield holder. The shoulders are gently tapped down to enhance visualization of the lower cervical spine with intraoperative lateral fluoroscopy. The segment(s) to be operated on can be identified by intraoperative C-arm fluoroscopy. In case of a two- or three-level procedure, a small (18–20 mm) transverse skin incision is recommended to be place at the midpoint of the operative distance. The prevertebral anatomical structures have the characteristics to be movable. Skin incision has to be made deep to the platysma before subplatysmal structures can be dissected by index and the middle finger. The larynx is pushed toward the opposite side with the index and middle fingers while muscle and the carotid was held laterally. Next fingers were slipped inside towards the front of the vertebral body until the anterior cervical spine and edge of the disc is palpated. Optional an artery forceps is placed through the skin incision between both fingers with its blunt tip kept at the vertebral leading edge by creating an access path. Next step the endoscopic tubular dilators were introduced sequentially under fluoroscopic guidance between the carotid artery and the esophagus. A working trocar with an outer diameter of about 18–20 mm is introduced at last and fixed to a mechanical flexible holding arm which is attached to the operating table. After confirming the correct level via lateral C-arm-fluoroscopy the dilators are removed and endoscope system of choice is installed. The anulus fibrosus of the disc is incised by using a microknife before the nucleus pulposus is removed. Osteopytes can be removed by Kerisson punch or diamond drill. Continuous irrigation with saline solution is recommended to remove remaining fragment and to prevent thermal nerve damage in case of drilling. An arterial or any kind of source of bleeding from paraspinal muscular can be controlled by bipolare forceps. Micrograsper, microforcep, dissectors and small curettes are used to remove the rudiment of the disc of the vertebral body. Special curettes are available to dissect the remnant cartilage endplates and enlarge the intervertebral space. Since distraction screws are not placed manual cervical distraction is performed to widen the interbody space by pushing up the head gently and pulling down the arms at the same time. Another technique for distraction is placing a 5 mm osteotome in the disc followed by its twisting. A cage of choice is placed under fluoroscopic guidance and optional filled with bone graft substitutes. After removal of the tubular retractor, the subcutaneous tissue is closed in standard fashion with a skin adhesive and steristrips the placement of a suctions drain is optional (Fig. 7.2).
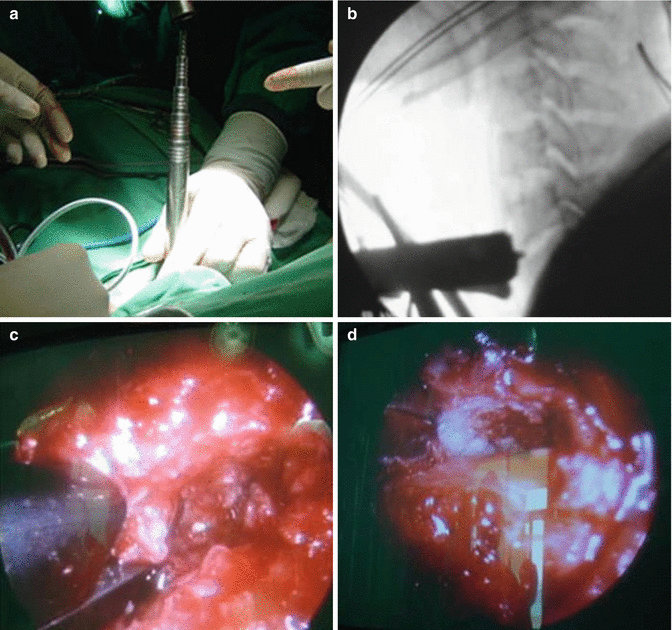

Fig. 7.2
Cervical Microendoscopic Discectomy and Fusion. (a) Sequential introduction of endoscopic dilation system between carotid artery and the esophageal. (b) Position of working trocar under fluoroscopic control. (c) Removal of anterior osteophyte via Kerrison. (d) Endoscopic view after implantation of intervertebral cage
Outcome
CMEDF offers similar functional outcome compared to ACDF
Odom’s criteria 86–91 % of patients report an excellent or good functional recovery
Significant decrease in arm- and neck pain on visual analog scale (VAS)
Significant improvement concerning Japanese Orthopaedic Association (JOA) score
Significant reduction in postoperative analgesic doses, and length of hospital stay
Low rate of laryngopharyngeal complications
Advantages of CMEDF
Reduction in the doses of postoperative analgesic
Reduction in length of hospital stay
Reduction in laryngopharyngeal complication
Better aesthetical result
Less retraction and manipulation at the trachea and esophageal
Complications (Access-Related)
Complications of the traditional open approach are possible
Vascular injury
Esophagus
Trachea
Thyroid
Laryngeal nerves
Anterior Percutaneous Endoscopic Cervical Discectomy
The standard treatment for cervical soft disc herniation in spine surgery is anterior cervical discectomy and fusion ACDF. The majority of surgeons perform ACDF because the theory behind it is that fusion prevents segmental instability and kyphosis due to reconstruction of empty disc space by implanting a graft out of an autologous iliac crest, a cage (e.g. PEEK, titan) or disk prosthesis. Surgeons are concerned that cervical alignment would be distorted due to a collapse of the operated segment without fusion which could result in axial neck pain and radicular arm pain in case of a compromised neuroforamen. In the past decades there was less discussion about the imperative of fusion. Little research about the outcome after ACD compared to ACDF although postoperative clinical results seems similar [45, 46]. Since the first description of cervical percutaneous discectomy by Tajima et al. many minimally invasive techniques were developed to treat cervical spine disease [47]. Anterior percutaneous cervical procedures for decompression of the nerval structures can be divided into techniques with endoscopic visualization and nonvisualized techniques. The objective of both techniques is to reduce the nerve compressing volume. Nonvisualized techniques can reduce the volume either via aspiration of the nucleus pulposus [48, 49], via radiofrequency [50, 51], or via radiofrequency [52]. The success of surgery depends on adequate decompression of the nerve root. Therefore the nonvisualized techniques are criticized for their lack of to identify free disc fragments and to assess the status of decompression intraoperatively. The anterior percutaneous endoscopic cervical discectomy (APECD ) combines the advantages of minimally invasive approach via a needle and the inspection of the intradiscal space via endoscopic visualization.
Further holmium: Yttrium-Aluminium-Garnet (YAG) laser can be used via this technique for decompressive and thermoannuloplasty. Although a percutaneous cervical stabilization with an expandable holder can be performed via this approach. The idea behind it is to maintain the disc height after decompression. This chapter will deliver an impression for the endoscopic technique and equipment that is necessary to perform APECD and gives a short review about clinical results.
Indications
The APECD approach in indicated in the following cervical pathologies and situations [41, 53–55]:
Unilateral radiculopathy with sensory disorder, reflex abnormality, motor weakness, arm pain with and intractable neck pain unresponsive to conservative management over 12 weeks
Magnetic resonance imaging (MRI) computed tomography (CT) that is positive for mediolateral localised monosegmental contained or noncontained soft disc herniation
Segments C2–3 to C7–Th1;
Ventral and posterior disc height must be least 4 mm
Contraindication
Osseous foraminal stenosis
Intraforaminal disc herniation
Calcified disc or disc height of less than 4 mm
Central canal stenosis with broad disc bulging
Craniocaudal dis sequestering of more than half of the vertebral body
Evidence of instabilities and / or deformities
Evidence of myelopathy
Isolated neck pain
Foraminal stenosis without disc herniation
Previous operation at the same segment
Surgical Equipment for APECD
The following surgical equipment and instruments are necessary to perform APECD [40, 41, 55]:
Endoscopic system (e.g. Karl Storz GmbH & Co KG, Tuttlingen, Germany) Outer diameter 4.0 mm/working length 12 cm/central working channel 1.9 mm
Video digital endoscopy unit with a camera
0° endoscopic optic
Special endoscopic instruments (microforcep, trepine etc.)
Discography : Telebrix (Guerbert, France), Contrast agent: Indigo carmine (Korean United Pharma, Seoul, Korea)
Holm-yttrium aluminium garnet YAG laser (e.g. Trimedyne, Inc., Irvine, CA)
Fluoroscopy (C-arm)
Surgical Technique
The patient can be operated on under local anesthesia with light sedation so the surgeon can talk to the patient and be aware of any neurological changes. However, if preoperatively there is a sign that the patient may suffer from intraoperative psychological or physical stress because of the introduction of the endoscopic system, general anesthesia can be used as an alternative instead. Preoperative antibiotics are admitted and dexamethasone might be administered to minimize airway and esophageal edema. The patient is placed in a supine position with the neck mildly extended on a radiolucent table. Intraoperatively fluoroscopic guidance (C-arm) the segment of operation is carefully identified in lateral and a.p. X-ray using a radiopaque instrument. Skin incision is marked and with a felt-tipped pen and the skin and subcutaneous tissue were infiltrated with local anesthetic (e.g. 1 % xylocaine, 1 % lidocaine). Generally the approach is at the contralateral side about 2–5 mm paramedian from the midline. The anatomical structures are very mobile due to the compartmentalization and ideal for an anterior percutaneous approach. The goa is to displace the trachea and esophagal medially and the carotid artery and internal jugular vein laterally. First the pulsation of the carotid artery should be felt, then the visceral structures (thyroid, trachea, larynx, and esophageal) can be mobilized to the opposite side with the index finger. The middle finger is then slipped inside towards the cervical spine till the protruding ring of the disc is felt between both plane forefronts of the vertebral bodies. Under continuous fluoroscopy a 18-gauge puncture needle is then carefully inserted in the space between the visceral and vascular compartment into the disc space close to the posterior body line of the posterior part of the disc, trying to preserve the longuscoli muscle. A next step of the surgery a discography (e.g. 10 mL Telebrix and about 0.5 mL of contrast media e.g. indigo carmine is injected to specify the posterior part of the disc) is performed to determine the annular tear, to confirm the presence of soft disc herniation, and to stain the nucleus pulposus into blue in contrast with the neural tissue. Then a guide wire is inserted to replace the puncture needle and is skin incision of 3 mm is made to allow the dilation of the skin and soft tissue via serial progressive dilator (2–5 mm). By this technique soft tissue is prevent from trauma and the approach-related pain is reduced. Finally, the tip of the working cannula is firmly placed to reach the posterior part of the disc. Its correct position sis confirmed via fluoroscopy. The distance between the tip of the working cannula and the end of the posterior longitudinal ligament represent the working depth for the endoscopic instruments. Next the endoscope is inserted into the working cannula. Continuous saline mixed with antibiotics (e.g. cefazolin) is used as irrigation. First endoscopic images of the intradiscal cavity are visible on a monitor. Under endoscopic visualization the herniated disc fragments are removed with microforcep and trephine without injuring the spinal cord. The risk of instability or local kyphosis may be avoided by leaving the anterior fraction of the disc intact while removing the posterior aspect. If necessary the endplate and parts of the posterior edge of the vertebral body are ablated via a Holmium yttrium–aluminum–garnet
(Ho:YAG) laser. Further the laser can be used to shrink the remaining disc herniation and to vaporize abnormal annular structures. A low doses of energy for laser with 2 J and 10 Hz is recommended, therefore YAG-laser is about 0.3–0.5 mm deep. The laser may be useful to create intradiscal cavity for exploration of adequate decompression. At the end of decompression posterior longitudinal ligament or dura should be clearly visible. Discoplasty of the working cannula surrounding disc tissue may be performed by YAG-laser before removing the working cannula. Position of the laser should be frequently checked via endoscope and fluoroscopy to prevent spinal cord or nerve root injury. Further an expandable holder or autologous iliac bone graft can be introduced for stabilization. The initial diameter of the holder is 3.3 mm while its inserted into the intradiscal cavity. It can be expanded up to 7.0 mm in diameter inside the disc by rotating the expander rotational handle. It is then fixed to the disc. The correct position is controlled under fluoroscopic guidance. If the procedure is performed under local anesthesia the surgeon can interact with the patient and ask whether the preoperative pain has disappeared or was relieved during surgery. The endoscope and working cannula were carefully pulled out. The remaining irrigation and blood is drained before the wound is closed in standard fashion (Fig. 7.3).
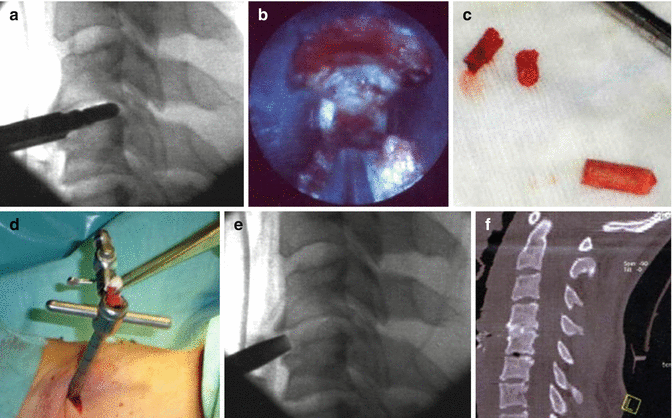

Fig. 7.3
Anterior Percutaneous Endoscopic Cervical Discectomy. (a) Fluoroscopic control of trocar and burr placement while preparation of the endplates. (b) Endoscopic view of the intervertebral space after preparation. (c) Autologous ilia bone graft (dowel shaped). (d) Inserting of bone graft in the working trocar. (e) Fluoroscopic control of the endoscopic placed bone craft. (f) Postoperative CT-scan confirms graft placement and fusion
Outcome
MacNab Criteria 83–91 % of patients report an excellent or good functional recovery
Significant decrease in arm- and neck pain on visual analog scale (VAS)
Significant improvement concerning Neck Disability Index (NDI)
Average time before returning to work 10–28 days
Significantly decrease in disc height
Postoperative development of segmental kyphosis and instability remains unclear
About 2 % of patients need additional open surgery
Advantages of APECD
Outpatient procedure
As safe as standard open technique
Complications (Access-Related)
Carotid artery injury (dissection, rupture)
Jugular vein
Postoperative temporary headache due to prolonged high irrigation pressure because of epidural venous bleeding
Decreased disc height
Discitis
esophagus,
Trachea,
Thyroid and
Laryngeal nerves
Endoscopic Posterior Cervical Laminoforaminotomy
In 1951 Frykholm first described posterior foraminotomy through partial resection of the medial margin of facet joint to decompress the cervical nerve root in a series of patients with radiculopathy [56]. At that time this new technique was a big step ahead of the established operative techniques for dorsal decompression of the cervical spine like laminectomy with or without chiseling of retrospondylophytes. Conventional posterior approaches have the disadvantage of the detaching the extensor cervical muscles from the laminae and the spinous process. Detaching of the paraspinal muscles can causes a severe trauma and can come along with postoperative complications like axial neck pain, shoulder pain, loss of lordosis or even spinal instability [57, 58]. The posterior cervical laminoforaminotomy technique applied microsurgical principles to the dorsal approach to the cervical spine for the first time. It enables bony decompression of the nerve root in cases of foraminal stenosis or removal of disc fragments with the advantage of far less injuries to the myelon. It does not allow the removal of medioventral nerve compressing lesions. Nonetheless through the development of the anterior approach to the cervical spine (ACDF) by Smith and Robinson and modified by Cloward in 1958 eluded the problem of the myelon being in the way of the pathology [34, 35]. As a consequence the anterior approach became the gold standard in the treatment of degenerative cervical disc disease and cervical stenosis for decades and the posterior approach became obsolete by the time. However, ACDF results in the loss of a motion segment, fusion and approach-related morbidity and graft-related complications. A widespread movement came into the development of new techniques for the treatment of degenerative cervical diseases. Besides alternative to segmental fusion in the anterior approach which mostly centred in artificial disc replacement, the posterior foraminotomy was rediscovered and improved upon [59]. The aim to reduce iatrogenic trauma related to the surgical approach has led to the evaluation of neuroendoscopy in the spinal surgery. Endoscopic lumbar discectomy has been shown to produce comparable results to standard microsurgical discectomy with the advantage of less muscular trauma and thereby less back pain [31]. However till today there is still no consensus about the ideal surgical approach for the treatment of cervical radiculopathy. Depending on the morphologic pathology advantages and disadvantages of both approaches and surgical techniques have to be kept in mind when deciding which approach is ideal. In cases, in which the cause of compression is located posterolaterally, such as intraforaminal cervical herniation, the posterior foraminotomy has showed to be effective and safe [60]. This chapter will deliver an impression of the posterior endoscopic cervical laminoformaminotomy (PECLF) technique and equipment that is necessary to perform PECLF and gives a short review about clinical results.
Indications
The EPCLF approach in indicated in the following cervical pathologies and situation:
Osseous foraminal stenosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








