Endovascular Revascularization of Carotid Artery Disease
Objectives: Upon completion of this chapter, the reader should be able to describe balloon angioplasty and stenting of atherosclerotic disease involving the carotid artery, as well as the numerous clinical studies that have defined the indications for endovascular therapy.
Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians.
Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity.
The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp
* The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons.
More than 750,000 people suffer a stroke annually in the United States costing an estimated $52 billion in treatment and lost productivity. This makes cerebrovascular disease the third leading cause of death in the United States. While there are many causes of stroke, carotid occlusive disease accounts for 25% of all strokes. Carotid stenosis affects 0.5% of Americans after age 60 and increases to 10% in persons >80 years of age, yet most cases remain asymptomatic. In this chapter, we will evaluate the evolution in the treatment of carotid stenosis using stent-supported angioplasty.
Surgical carotid endarterectomy has historically enjoyed an unchallenged role in the treatment of carotid stenosis and remains the accepted standard of care for revascularization of extracranial carotid occlusive disease. Such a pre-eminent position is relatively rare in medical practice and has been validated by multiple, randomized, controlled trials demonstrating its efficacy over medical therapy. Any challenger will have to prove efficacy over and above this well-characterized methodology.
During the past 24 years, endovascular techniques for the treatment of cerebrovascular atherosclerotic disease have undergone technical advancement and increasing clinical application. Starting with balloon angioplasty alone, percutaneous revascularization procedures have progressed to the use of metallic stents for improved immediate and long-term results. Stent-supported angioplasty now offers a therapeutic alternative to traditional methods of surgical revascularization and treatment for those individuals without surgical options who have failed maximal medical therapy. Additionally, endovascular techniques offer treatment for a variety of non-atherosclerotic diseases affecting the craniocervical arteries including inflammatory, radiation-induced, and postsurgical strictures; acute intimal dissection; traumatic and spontaneous arteriovenous fistulas; aneurysms; and pseudoaneurysms.
For patients at high risk for surgical complications, endovascular procedures have gained clinical, if not scientific, preference as the therapeutic modality of choice. Continued innovation and refinement of endovascular technology and techniques will further improve technical success, reduce procedural morbidity, and broaden the endovascular therapeutic spectrum for extracranial and intracranial cerebrovascular disease. The first NIHsponsored trial to compare carotid endarterectomy with stent-angioplasty (CREST) has now begun enrollment. In time, stent-angioplasty may supersede endarterectomy as the procedure of choice to treat carotid stenosis.
 Endovascular Treatment of Carotid Occlusive Disease
Endovascular Treatment of Carotid Occlusive Disease
Carotid Balloon Angioplasty
Kerber is often credited with the first reported case, in 1980, of percutaneous transluminal balloon angioplasty for carotid artery stenosis.1 Seven years later, Theron published a study of 48 patients treated for de novo atherosclerosis or postsurgical restenosis using carotid angioplasty. Technical success was achieved in 94% of cases, with a 4.1% rate of serious morbidity.2 By 1995, 96.2% technical success had been demonstrated in 523 carotid angioplasty procedures with 2.1% permanent morbidity, 6.3% transient minor complications, and no deaths.3 In 1996, Gil-Peralta described 85 balloonangioplasty procedures in 82 patients with symptomatic carotid stenoses greater than 70%. The results showed a 92% technical success, 0% 30-day mortality, and 4.9% major morbidity, which compare very favorably to the European Carotid Surgery Trial (ECST) and the North American Symptomatic Carotid Endarterectomy Trial (NASCET.)4 Restenosis at 18.7 months follow-up occurred in 6.7%, predominantly between 3 to 6 months post treatment, and was entirely asymptomatic. Restenosis within 2 years of treatment has been reported in other large angioplasty series and ranges between 0 to 16%.5,6 Meanwhile, carotid endarterectomy series report 10% early restenosis.7
Limitations to balloon angioplasty include vessel wall recoil, intimal dissection, and plaque disruption with particulate embolization. Angioplasty of atherosclerotic lesions generates emboli composed of atheroma, cholesterol crystals, thrombus, and platelet aggregates.8,9 Similarly, embolization of microparticles has also been shown during and after carotid endarterectomy correlating with complex plaque morphology10 and with clinical postoperative cerebral ischemia.11
Studies examining the frequency of emboli during carotid balloon angioplasty using transcranial Doppler (TCD) have failed to show a clear-cut correlation between number of insonated emboli and neurological sequelae.9 By using transcranial Doppler ultrasound in 28 patients evenly divided between carotid angioplasty and endarterectomy, Crawley reported an average of 202 embolic signals during carotid balloon angioplasty compared with 52 during carotid endarterectomy. During a 20-minute postoperative recovery period, the tables turned and the average number of embolic signals was 5 for balloon angioplasty versus 19 for endarterectomy. However, no correlation was found between the number of high-intensity transient signals (HITS) and the rate of periprocedural stroke.8
The risk of stroke from embolic debris is thought to depend upon size and composition as well as the extent and location of brain involvement. It is difficult to accurately distinguish between air and particulate emboli using TCD12; therefore, the lack of correlation between emboli and clinical sequelae has led to the suggestion that the large majority of emboli detected during balloon angioplasty are either gaseous or small platelet aggregates >200 μm in diameter, both of which correlate with a more benign outcome.8 Premedication with antiplatelet agents to prevent larger platelet aggregates is now commonly endorsed.13
Endovascular revascularization of carotid occlusive disease may result in cerebral hypoperfusion from luminal compromise by catheters and guidewires crossing the stenotic lesion and/or during balloon inflation. This is of even greater relevance in the presence of contralateral carotid artery occlusion or stenosis. Eckert monitored 22 patients undergoing carotid balloon angioplasty using TCD, noting that a >50% reduction of middle cerebral artery mean blood flow velocity compared with baseline values represented a critical threshold for the development of ischemic symptoms in conscious patients, even with short occlusion times of 10 to 40 seconds.14 By contrast, Crawley reported significantly greater hemodynamic ischemia during endarterectomy than balloon angioplasty.8 Consequently, balloon inflation and occlusion times are commonly brief (less than 30 seconds) in these circumstances to avoid the risk of potential cerebral ischemia. If there is attendant compromise of the contralateral carotid artery, assessment of the cerebral collateral circulation becomes even more important, and the procedure should be performed with minimal sedation to facilitate neurological monitoring.
Stent-Supported Angioplasty
The impetus for carotid stenting has arisen principally from the descriptions of the efficacy of stent-supported angioplasty in the coronary circulation that has consistently demonstrated persistent benefit in eventfree survival at 1 year and a reduced need for repeat angioplasty.15 The theoretical advantages of stentsupported angioplasty over angioplasty alone include reduction in symptomatic plaque embolization, intimal dissection, elastic vessel recoil, and early and late restenosis. Although it remains unknown whether or not these benefits apply to the carotid circulation, endovascular carotid revascularization is now most commonly performed with stents.
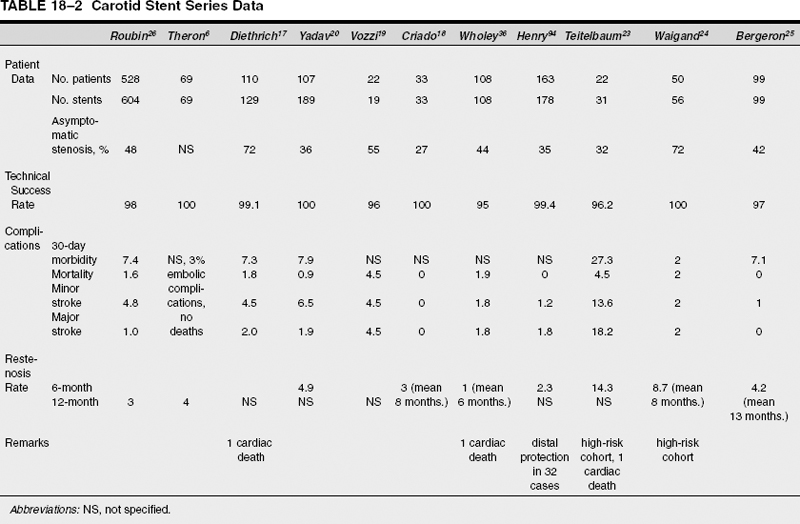
Since 1996, there have been 11 large carotid stent series published in which the total number of patients is 1311.6,16–26 Comparative analysis of these reports is difficult due to inconsistencies in the sample populations, lesion characteristics, endovascular techniques, and outcome data. Overall, technical success is >95%; procedural mortality rates including cardiac deaths are 0.6 to 4.5%; major stroke rates are 0 to 4.5%; minor stroke rates are 0 to 6.5%; and the 6-month restenosis rate is >5%. This excludes other studies on high-risk patient populations.27–30 Similarly, favorable results were reported by Al-Mubarak on 51 patients undergoing simultaneous or staged carotid artery stenting and percutaneous coronary intervention. Technical success was achieved in all carotid arteries with 4% minor stroke and no major strokes, myocardial infarctions, or deaths.31 In NASCET, the perioperative stroke and death rate was 5.8%, with 0.6% mortality predominantly due to myocardial infarction.32
Vitek reviewed a series of 404 patients treated for carotid stenosis using stent-angioplasty with 98% technical success, 1.9% 30-day morbidity–mortality, 0.7% major stroke rate, 5.8% minor stroke, and 5% restenosis.33 Lower complication rates were noted in the last 122 patients, presumably due to increased operator experience with 2.5% minor stroke and no major strokes or deaths. Finally, Wholey published the results of a worldwide stent survey in which 3047 carotid stent procedures from 24 centers in America, Europe, and Asia, with 0.98% 30-day procedural mortality, 1.35% major stroke, 2.53% minor stroke, 2.23% restenosis at 6 months, and 2.48% restenosis at 12 months.34 Centers performing fewer than 50 cases experienced 6.4% major stroke and death, compared with 2.3% for centers performing between 50 to 100 cases. Consequently, Wholey suggests a 50-case learning curve for carotid stent angioplasty.
Operator experience appears to have an important role in treatment outcome.35 It is evident that some threshold for excellent outcomes exists but the exact training and experience the treating physician must have remains to be determined. Furthermore, the impact of case performance on complication rates must be clarified. For centers that performed fewer than 50 cases, there was a 5.9% rate of major stroke and death. By comparison, in those centers with a throughput of 50 to 100 cases, the complication rate fell to 2.6%, while a complication rate of 1.7% was found at centers contributing 100 to 200 cases. Interestingly, participating centers with 200 to 300 and 300 to 400 cases reported increasing periprocedural complications.35
Treatment outcomes for NASCET-ineligible patients (ie, those considered higher risk for surgery) undergoing stent angioplasty have been studied. In NASCET, the perioperative stroke and death rate was 5.8%.32 NASCET criteria were applied to patients enrolled in five carotid stent series demonstrating that 79% of these 574 patients would have failed NASCET eligibility because of medical comorbidities.16,20,24,36 (Fig. 18-1). Nevertheless, 2.0 to 7.9% morbidity and 0.6 to 2.0% mortality in these early stent series compare favorably both with NASCET and ECST. Still, 41% of these patients had asymptomatic carotid stenosis for which lower endarterectomy morbidity and mortality rates might be expected. For another group of NASCET ineligible patients, Yadav reported 22 patients treated for postendarterectomy carotid stenosis, with stent angioplasty resulting in no major and only one (4.5%) minor stroke.37 Lanzino reported favorable results in 18 patients undergoing stent angioplasty for recurrent stenosis following endarterectomy with no periprocedural strokes and one transient ischemic attack.38 Mericle described 23 patients with high-grade carotid stenosis and contralateral carotid occlusion treated electively using stent angioplasty with no 30-day perioperative stroke or deaths.39 In NASCET, the perioperative stroke and death rate in the presence of contralateral carotid occlusion was 14.3%.32
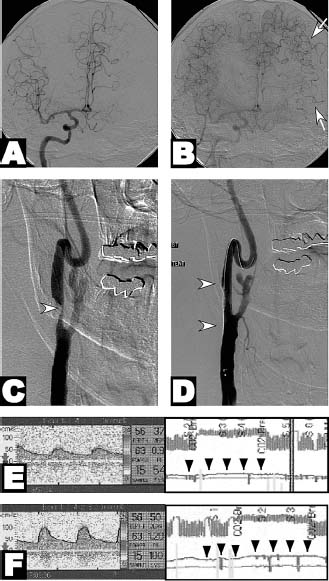
FIGURE 18-1 A 70-year-old man presented with known left carotid occlusion developed episodic right hemiparesis and expressive aphasia. (A, B) Right common carotid arteriography in the frontal projection during early and late arterial phases demonstrates that the left middle cerebral artery distribution depends predominantly upon leptomeningeal collaterals (arrows) from the anterior cerebral artery circulation. (C, D) Right common carotid arteriography demonstrates 95% eccentric stenosis (arrowhead) of the right internal carotid artery origin treated with an 8 × 30 mm nitinol stent. (E, F) Transcranial Doppler sonography before (E) and after (F) inhalation of 5% carbon dioxide demonstrates dramatic improvement in cerebral vascular reactivity (arrowheads) associated with improved cerebral blood flow. This method has been shown to be a strong predictor of cerebral hemodynamic failure and stroke.109
Mathur retrospectively analyzed stroke risk factors in 231 patients undergoing elective stenting of the extracranial carotid arteries in which only 14% of patients were NASCET eligible.40 The overall 30-day stroke rate was 6.9%, while the comparable stroke rate was only 2.7% in the NASCET-eligible group. Advanced age beyond 80 years, long or multiple stenoses were found to be independent predictors of periprocedural stroke. Contralateral carotid occlusion, prior carotid endarterectomy, and combined carotid and coronary procedures, all of which are associated with a higher incidence of complications in carotid endarterectomy, did not result in an increased rate of adverse outcome in stent patients.40,41 Chastain also found that age was a risk factor for carotid stent angioplasty. One hundred eighty-two patients treated with carotid stent angioplasty were stratified into three age groups: >80 years; 75 to 79 years; ≤74 years. Neurological complications were significantly more frequent in patients >80 years (25%) than in patients ≤74 years (8.6%). Major stroke and death occurred in 1.6%, with a 0.5% rate of myocardial infarction.42
There are few published studies directly comparing carotid endarterectomy with carotid stenting. Jordan retrospectively compared 107 endarterectomy patients with 166 prospectively treated stent angioplasty patients and found a higher early minor stroke rate in the stent group (6.6% vs. 0.6%) but a greater risk of major stroke and death in the surgical cohort (4.2% vs. 2.8%).43 Jordan compared outcomes from a subset of 109 endarterectomy patients treated under regional anesthesia with 268 stent angioplasty patients at the same institution. The early stroke and death rate was greater in the stentangioplasty group: 9.7% versus 0.9%. Yet, most strokes were minor. The major stroke rates between the two groups were more similar: 1.5% stent versus 0% endarterectomy. Naylor reported an early prospective, randomized study in 17 patients treated for symptomatic stenoses of >70% with no complications in 10 endarterectomy patients, while five of seven stent patients suffered strokes, three of which remained disabling at 30 days.44 This trial was prematurely terminated in favor of endarterectomy; methodological problems may explain the unusually disappointing results associated with stent angioplasty.
In general, review of the current literature on carotid stent angioplasty reveals technical success rates, procedure-related morbidity and mortality, and restenosis rates comparable with the results of endarterectomy.45–47 Unfortunately, inconsistencies in trial design, reporting criteria, and follow-up render direct comparison of endarterectomy with endovascular revascularization difficult (Table 18-1). The validity of comparisons between such disparate clinical data are unsound. In the case of carotid endarterectomy, there is Class I evidence of efficacy, whereas the bulk of the endovascular data are derived from nonrandomized, uncontrolled single institution data.48 For a more direct comparison, five prospective, randomized multicenter trials comparing carotid endarterectomy with stent angioplasty have been undertaken thus far.
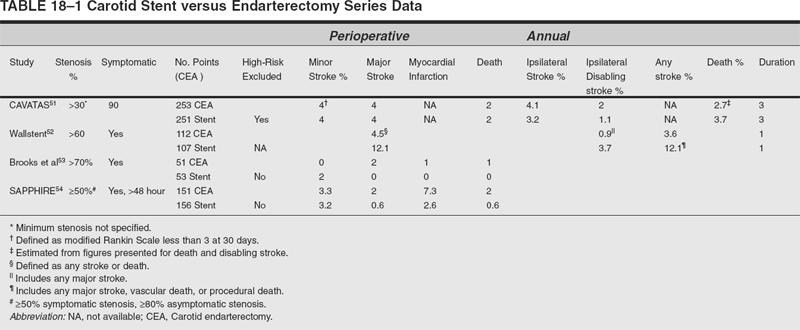
The Carotid and Vertebral Transluminal Angioplasty Study (CAVATAS) was a prospective, randomized, multicenter trial including 504 patients randomized to endarterectomy, stent angioplasty, or medical therapy.49 Patients with symptomatic stenoses (at least 30% luminal diameter reduction) suitable for surgery were randomized between endarterectomy and stent angioplasty over 5 years. Patients unsuitable for endarterectomy were randomized to stent angioplasty or medical treatment alone. The findings are remarkable: There was no significant difference in procedural risk of stroke or death between endarterectomy and stent angioplasty. The rate of any stroke lasting more than 7 days or death within 30 days of first treatment was nearly 10%, while the rate of disabling stroke or death within 30 days of first treatment was 6% in both surgical and endovascular groups. Preliminary analysis of long-term survival showed no difference in the rate of ipsilateral stroke or any disabling stroke in patients up to 3 years after randomization.49 In CAVATAS, the 30-day stroke or death rates are higher than those in many prior studies but are not significantly different from the 7% stroke and death rate in ECST.50 Cranial nerve injury and myocardial ischemia were only reported at the time of treatment in the endarterectomy group but not thereafter. Long-term follow up beyond 3 years is not yet available.51
The Carotid Artery Stenting versus the Carotid Endarterectomy trial (the Wallstent Trial) was an industry supported prospective, randomized trial comparing endarterectomy and stent angioplasty for treatment of symptomatic stenosis ≥60%. The results were disappointing and were only published as an abstract.52 In this study, 219 patients with symptomatic 60 to 90% carotid stenosis were randomized to endarterectomy or stent angioplasty. The perioperative risk of stroke or death was 4.5% for surgery but 12.1% for stent angioplasty. At 1 year, the major stroke rates were widely disparate at 0.9% for surgery versus 3.7% for stent angioplasty. Consequently, this trial was prematurely halted due to poor results from stent angioplasty.
Brooks reported a single community hospital’s experience randomizing 104 symptomatic patients to either carotid endarterectomy or stent angioplasty without distal protection.53 Perioperative stroke or death was 2% for endarterectomy and 0% for stent angioplasty. Other endarterectomy complications totaled 16% including hematoma requiring treatment, cranial nerve injury, and hypotension requiring treatment. Stent angioplasty complications totaled 45% including transient cerebral ischemia, leg amputation, retroperitoneal hemorrhage, bradycardia requiring temporary pacing, and symptomatic hypotension
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial randomized 307 high-risk patients to endarterectomy or stent angioplasty using distal protection. High risk was defined as congestive heart failure (class III/IV) or ejection fraction >30%, open-heart surgery needed within 6 weeks, recent myocardial infarction (24 hours to 4 weeks), or unstable angina (CCS class III/IV). Among randomized patients, the cumulative risk for stroke, death, or myocardial infarction at 30 days was 39% lower with stenting than endarterectomy. The combined risk for the two primary endpoints at 1 year was 7.9% lower with stenting. The authors concluded that stenting with embolic protection is not inferior to endarterectomy (p =0.004). The data narrowly missed the mark for statistical superiority with stenting (p =0.053). Moreover, fewer patients with stents required reoperation than those who underwent endarterectomy.54
However, the SAPPHIRE trial remains controversial. Of enrolled patients, 55% were excluded from randomization as poor surgical candidates, a number that appears high and may introduce bias. More than 20% of randomized patients were being treated for restenosis following prior endarterectomy, likely favoring the endovascular approach. Finally, myocardial infarction was included in the composite endpoint; hence, the effects of less-frequent strokes and deaths were obscured, which were the primary endpoints in previous large endarterectomy trials. The SAPPHIRE trial was not powered to evaluate stroke and death alone.
The Carotid Revascularization Endarterectomy versus Stent Trial (CREST) is the National Institute of Health– funded North American, multicenter, randomized, controlled trial comparing the efficacy of surgical endarterectomy with carotid stenting.55 In CREST, patients with symptomatic extracranial carotid stenosis of ≥50% are randomized to stent angioplasty or endarterectomy. To demonstrate a clinically significant difference between the two treatments, CREST will require at least 2500 patients. To evaluate for differences among clinical subgroups, such as groups affected by recurrent stenosis, an even greater number will be required. Predominantly because of political and contractual issues, the trial has been slow to enlist centers ready to enroll patients, thus risking withdrawal of government funding for this endeavor.
Additional multicenter trials will help to provide supporting evidence for the role of stent angioplasty. Currently, there are three other major trials in progress. EVA-3S is a French trial randomizing 900 patients with ≥70% (by NASCET criteria) symptomatic atherosclerotic carotid stenosis to surgery or stentangioplasty.32 The Stent-Protected Percutaneous Angioplasty of the Carotid versus Endarterctomy (SPACE) trial is a German study comparing 950 patients in both endarterectomy and stent-angioplasty groups with symptomatic carotid stenosis measuring ≥50%.56 The International Carotid Stenting Study (ICSS) is a multinational study undertaken on four continents that will randomize 2000 patients with symptomatic carotid stenosis measuring ≥70% by common carotid measurement method to endarterectomy or stent angioplasty as a randomized follow-on trial of CAVATAS.57
Procedural Technique
Depending on existence of comorbidities, medical therapy may need to be adjusted before arteriography and intervention. Patients on long-term oral anticoagulation should be converted to heparin. Patients with pre-existing renal disease may be admitted on the prior day for intravenous hydration or monitored vasodilator therapy. Oral entericcoated aspirin and clopidogrel (Plavix Bristol-Meyers Squibb, New York, New York) have been recommended at least 3 days before the procedure to reduce periprocedural platelet emboli. Experimental data suggest aspirin and clopidogrel have a synergistic effect on platelet aggregation, thrombotic activity, and prevention of restenosis due to fibromyointimal proliferation.58 Following the procedure, oral antiplatelet agents are usually continued. We usually will continue Plavix for 6 weeks and aspirin indefinitely.
Although the procedure may be performed under general anesthesia, stent angioplasty of the cervical carotid artery is most commonly performed under local anesthesia at the puncture site with or without light conscious sedation which allows continuous monitoring of the patient’s neurological status.59 While the decision to proceed with treatment may have been made based on prior noninvasive imaging or arteriography, the final determination of stenosis severity is made based upon the arteriogram at the time of intervention. Carotid stenosis may be eccentric; therefore, the optimal projection to demonstrate the stenosis should be found, which may require multiple projections or even rotational angiography.60 In the case of presumed ICA occlusion, prolonged filming is necessary, as otherwise delayed, faint, antegrade opacification due to an angiographic “string sign” of critical ICA stenosis may be missed.61 Standard anteroposterior (AP) and lateral intracranial views should be obtained in all cases to establish the adequacy of the intracranial collateral circulation via the external carotid and anterior communicating arteries, and also to document any distal intracranial stenotic lesions. Many experienced operators also advocate routine vertebral angiography to assess collateral flow via the posterior communicating arteries and to document any extracranial or intracranial vertebrobasilar stenoses. Certainly, in cases of contralateral carotid occlusion, bilateral severe stenosis, or deficient anterior collateral pathways, evaluation of the adequacy of collateral flow via the posterior circulation becomes even more important.
The use of systemic heparin is standard. Angiomax (bivalirudin, The Medicines Company, Parsippany, New Jersey), a newer alternative to heparin, may have some preferable properties.62 Glycoprotein IIb/IIIa inhibitors such as abciximab have been shown to decrease mortality and morbidity in several coronary stent studies and also potentially improve long-term patency rates, but their role remains undefined in carotid artery stenting.63 These agents may have a potential role in the uncommon event of acute stent thrombosis because white thrombus (platelet aggregates) represents the primary mechanism of thrombosis.64
The decision to pre-dilate the lesion using balloon angioplasty depends upon the type and size of stent being used, the narrowest lumen diameter, and the morphological configuration of the stenotic segment. Many operators perform routine pre-dilatation to ~2.5 to 4.0 mm diameter. However, others suggest that the risk of embolism is high during this part of the procedure and if atraumatic crossing of the lesion with a filtration device or the stent-delivery-catheter is possible without pre-dilatation, then this may be preferable.
The currently used stents are either balloon-expandable or self-expanding, with the self-expanding stents made of either stainless steel or Nitinol (nickel-titanium alloy). Early reports of carotid stenting mainly utilized balloonexpandable stents such as the Palmaz, or the newer Genesis stent (Johnson & Johnson Interventional Systems Co., Warren, New Jersey), which allow greater precision than self-expanding types. Balloon-expandable stents are not optimal for cervical carotid revascularization because of reports documenting a 2 to 16% rate of Palmaz stent collapse on follow-up imaging, which has led to increased use of self-expanding types.65 Furthermore, carotid Doppler ultrasound performed retrospectively was only 29% sensitive at identifying seven patients with stent compression.65 In Wholey’s global survey of 3047 carotid stenting procedures including 3033 endovascular carotid stents, balloonexpandable Palmaz stents were used in 47% of cases, and all 28 stent deformations occurred exclusively with the Palmaz stent.65 However, Bergeron reported no instances of compression at a mean 13-month follow-up among 96 patients with carotid stenosis all treated with Palmaz stents.25 Similarly, compression of balloon-expandable stents has been found to be a significant cause of restenosis in the superficial femoral arteries and hemodialysis grafts.66 Thus, the balloon-expandable stent may not be an ideal choice for superficially exposed arteries such as the internal carotid. For nonexposed locations such as the vertebral artery or great vessel origins, the superior positional deployment accuracy of balloon-expandable stents may be advantageous. Self-expanding Nitinol stents offer the purported advantage of crush recoverability due to their “spring-like” behavior. If an external force compresses or deforms a Nitinol stent, it will resume its expanded shape upon removal of the external stress.
In terms of stent sizing, the stent margins should optimally extend 1 cm beyond the proximal and distal margins of the stenotic plaque. This may necessitate “caging” or “jailing” of the external carotid artery origin by the stent mesh, which does not usually result in vessel occlusion or significant clinical sequelae. The stent diameter should be at least 1 to 2 mm larger than the largest vessel diameter the stent will need to appose. Stent oversizing leads to a greater metallic coverage of the lesion per unit area, which is theoretically advantageous in preventing distal embolism and reducing tissue prolapse through the stent and does not appear to be associated with a higher rate of restenosis.67
Postdeployment angioplasty using a high-pressure (12 to 20 atm), semi-compliant balloon may then be performed to closely appose the stent and intima, and moreover, to expand regions of residual stent narrowing. This may require the use of varying diameter balloons, particularly if the stent extends into the common carotid artery. Obvious gaps between the stent circumference and endoluminal surface potentially increase the risk of acute or delayed thromboembolism.68 Angioplasty beyond the stent margins is not recommended because this may cause acute vessel dissection, symptomatic vasospasm, or subsequent restenosis beyond the stent margins. Some operators do not advocate routine postdeployment angioplasty of the stent other than for obvious gaps between the stent and vessel wall and regions of residual stenosis, suggesting this reduces the risk of embolic complications and improves restenosis rates because of reduced intimal injury.
What defines technical success remains controversial. There is insufficient information to define technical success scientifically. For extremity and renal angioplasty, technical success requires >30% diameter residual stenosis by angiography and may require improvement in transstenotic pressure gradient.69 In the coronary literature, technical success for balloon angioplasty and stenting had originally been defined as at least 20% relative improvement with a decrease in stenosis to less than 50%, but it has recently been revised to a decrease in stenosis to less than 20% diameter.70 However, unlike extremity, renal, or coronary stenoses, carotid stenoses are very rarely symptomatic due to hemodynamic compromise. Rather, symptoms usually arise first from thromboembolism formation on carotid plaque with distal embolism.
It is unknown what degree of correction of carotid stenosis is necessary to reduce the risk of embolization, but removal of the embolic source is fundamental to procedural success. During stent angioplasty, it is possible that an attempt to eliminate residual stenosis more completely by complete balloon dilation can liberate additional emboli during the procedure that could cause a higher risk of procedural complications. Alternatively, severe residual stenosis may lead to a higher risk of late restenosis, which remains of uncertain clinical significance. Some carotid stenting trials have defined technical success as residual stenosis >30%.54 Others have used a definition of residual stenosis >50%.71 By recent consensus, technical success is arbitrarily defined as stent placement resulting in improvement of the stenosis by >20% with a final residual stenosis >50% using NASCET measurement criteria.72
Iatrogenic vasospasm may occur during the procedure, but it usually resolves shortly after removal of the guidewire from the internal carotid artery. Some investigators describe the use of injectable nimodipine (200 μg diluted in 10 ml injected slowly as a 2 to 3 ml bolus)68 or nitroglycerin (100 μg),36 and verapamil (5 mg diluted in 5 cc saline injected as a 1 to 2 mg bolus)73 into the carotid artery to treat mechanically induced vasospasm. Recalcitrant iatrogenic vasospasm usually responds well to low-pressure balloon angioplasty (>3 atm).
Anteroposterior and lateral cerebral angiograms are obtained following stent angioplasty to exclude any embolic branch occlusion and document changes in cerebral blood flow. Heparin anticoagulation is generally allowed to taper physiologically rather than be reversed using protamine sulfate. The arteriotomy may be closed immediately after the procedure with one of several percutaneous closure devices. Some operators have experimented with “outpatient” stent angioplasty of the extracranial carotid artery; however, patients are usually monitored in an intensive care setting for 12 to 24 hours post treatment. Antiplatelet agents are usually continued post procedure, currently including clopidogrel for 4 to 6 weeks and enteric-coated aspirin indefinitely.
The patient should be carefully examined neurologically following the procedure. Neurological deficits may be due to intracranial embolism, hemorrhage, or reperfusion injury.74 Following successful revascularization, reduction in mean arterial pressure 10 to 20% below baseline may be desirable to prevent cerebral hyperperfusion injury.74 Intravenous diltiazem (Cardizem, Bioval Pharmaceuticals, Mississauga, Ontario) may be used post procedurally to control elevated blood pressure, particularly if associated with headache or neurological sequelae, as it has minimal cerebral vasodilatory effects.75 Clonidine (Catapres, Boehringer-Ingelheim, Ingelheim, Germany) has been used in an effort to control the sympathetic autonomic neuronal pathways thought responsible for the hypertensive response following carotid baroreceptor injury.76 Metoprolol (Lopressor, Novartis Pharmaceuticals, Basel, Switzerland), or another β-blocker, may be useful to control the carotid pulsatility index following stent angioplasty.77 Prolonged postprocedural bradycardia or hypotension may occur as a result of carotid sinus dysfunction necessitating the use of intravenous vasopressors or inotropic agents,78 possibly even cardiac pacing.79
Posttreatment Evaluation
Posttreatment evaluation should include periodic evaluation for recurrent carotid stenosis. Recurrent carotid stenosis following endarterectomy is usually asymptomatic, but repeat revascularization is necessary in ~5% of patients who undergo carotid endarterectomy.80 Similarly, repeat endovascular revascularization for restenosis has been reported in 8% of patients undergoing carotid stenting,81 although the rate may be higher in patients for whom the original carotid stent was placed to treat a recurrent stenosis due to endarterectomy.82 Follow-up evaluation is easily performed with carotid duplex ultrasound and should be obtained at least 2 to 4 weeks post procedure, 10 to 12 months post procedure, and if ischemic symptoms recur. More frequent surveillance intervals may be useful in certain circumstances.83
 Complications
Complications
Stroke is both a complication of carotid revascularization and an outcome measure of the effectiveness of the revascularization. The stroke may be ipsilateral or contralateral to the treated vessel. Neurological deficits as a complication of the procedure may be due to intracranial embolism, hemorrhage, or reperfusion injury.84 The phenomenon of reperfusion hemorrhage following surgical endarterectomy is well described. Ouriel in a review of 1471 patients undergoing surgical endarterectomy reported a 0.75% incidence of intracerebral hemorrhage. Hemorrhage occurred at a median of 3 days postoperatively and accounted for 35% of the total perioperative neurological events. Death from massive hemorrhage and herniation occurred in 36% of cases. Factors correlating with an increased hemorrhage risk were hypertension, high-grade ipsilateral stenosis, highgrade contralateral stenosis or occlusion, and younger age.85 This complication has been reported with carotid angioplasty as well. Schoser reported two patients with reperfusion hemorrhage—one intraparenchymal hemorrhage and one subarachnoid hemorrhage following balloon angioplasty for carotid and vertebral artery stenosis.84 In a high-risk patient cohort, we found an unusually high 5% incidence of cerebral hyperperfusion phenomenon poststent angioplasty requiring intensive medical management to prevent sequelae such as seizure, stroke, and hemorrhage.74
Minor, major, and disabling stroke have not been uniformly defined in previous studies of endarterectomy or carotid stenting (Table 18-2). Other major complications include myocardial infarction and death, which in combination with stroke, are the major adverse events used to compare carotid stenting and endarterectomy. Some complications are unique to endovascular as compared with surgical revascularization. Carotid stent angioplasty may cause severe bradycardia and hypotension because of carotid sinus dysfunction requiring treatment. 78 Endovascular revascularization may produce vascular injuries such as dissection, perforation, hematoma, pseudoaneurysm, and groin infection. By contrast, complications unique to surgical revascularization include cranial nerve injuries, local wound hematomas, and infections.
 Costs
Costs
Retrospective studies examining the cost of endarterectomy versus stenting have indicated lower costs for their respective procedures.86–88 Surgical procedures include the costs of anesthesia and operating room time, while endovascular procedures include costs for catheters, stents, and angiographic room time. Cost-effectiveness analysis includes costs with respect to outcomes and may favor endarterectomy or stent angioplasty depending on major and minor complication rates.89 Randomized, prospective data are needed to accurately estimate this cost differential based on patient outcomes.
 Future Developments: Making Stent Angioplasty Safer
Future Developments: Making Stent Angioplasty Safer
Potential areas of innovation include cerebral protection using intravascular filters or temporary occlusion balloons; smaller diameter, more flexible, and hence, lesstraumatic stent delivery systems; reduction of restenosis from fibromyointimal hyperplasia by using catheter brachytherapy, radiation-emitting stents,90 or biologically active coatings,91 and improved adjuvant pharmacological regimes utilizing antiplatelet agents such as the glycoprotein IIb/IIIa inhibitors, which could reduce the risk of acute thromboembolism and improve long-term patency.92 The potential for future technical and pharmacological improvement has prompted some investigators to suggest that a direct comparison of stent angioplasty with endarterectomy may be premature.
Distal embolization during carotid angioplasty and stenting has generated tremendous interest and financial speculation in methods of cerebral protection (Fig. 18-2). A triple-coaxial catheter system, designed to provide cerebral protection, has been described by Theron,93 who reported distal embolic complications in 3 of 38 patients (8%) undergoing internal carotid artery balloon angioplasty without distal protection versus 0 of 43 patients (0%) undergoing the same procedure using distal balloon protection.68 However, Henry used Theron’s distal occlusion balloon technique in 32 of 163 carotid stenting cases in which 2 of the 3 major strokes occurred in conjunction with use of this device. Henry cited prolonged procedure time and increased embolic risk when traversing ulcerated lesions as potential problems associated with use of this particular system.94 Albuquerque employed a similar technique using distal balloon protection for the angioplasty portion of the stent-angioplasty procedure during 17 procedures on 16 patients with 94% technical success, 1 (6%) transient neurological complication, and no major strokes.95