20 Endovascular Therapies for Cerebral Revascularization
Introduction
Stroke is the third leading cause of death in the United States and the second leading cause of death worldwide. The AHA estimates that the direct and indirect costs of stroke in the United States in 2009 at $69.9 billion.1 Stroke has been recognized as a clinical entity for millennia, dating at least to ancient Greece. Hippocrates first described and documented the syndrome of stroke, which he called “apoplexy” (from the Greek apoplēssein, to strike down and incapacitate). Prior to the advent and widespread accessibility of high-resolution cross-sectional imaging, neurologists and neurosurgeons could only establish the location of strokes on the basis of clinicopathological correlation. Little was known about the pathophysiology of stroke for much of the 20th century, with in situ thrombosis being the presumed etiology for most ischemic strokes.2 The recognition of thromboembolic phenomena to the brain originating from the carotid artery and the description of lacunar syndromes allowed the development of strategies for stroke prevention.3,4 However, therapies for acute stroke were not developed until the latter part of the 20th century.
The first three trials evaluating the use of intravenous streptokinase for the treatment of acute stroke were terminated prematurely due to an increase in mortality rate, primarily related to the hemorrhagic conversion of ischemic infarcts.5,6 The European Cooperative Acute Stroke Study (ECASS),7 which tested recombinant tissue plasminogen activator (r-tPA) at a dose of 1.1 mg/kg, demonstrated no overall benefit to the use of intravenous (IV) r-tPA for the treatment of acute stroke. Subgroup analysis, however, revealed significant improvement after IV t-PA administration in patients with moderate to severe stroke without extended signs of infarction on CT. This study showed the therapeutic potential for thrombolytic agents in the treatment of acute stroke while underscoring the need to refine indications and contraindications for therapy.
The first trial to demonstrate significant clinical benefit for intravenous r-tPA with an acceptable risk of intracranial hemorrhage was the National Institute of Neurological Diseases and Stroke (NINDS) study.8 The NINDS study used a lower dose of intravenous r-tPA (0.9 mg/kg), which was administered within 3 hours of stroke onset. The results of the NINDS study led to the approval of intravenous r-tPA by the United States Food and Drug Administration (FDA) for use in acute stroke within 3 hours of onset of stroke symptoms. The safety and efficacy of the 3-hour therapeutic window was confirmed by the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) trial.9 The ATLANTIS trial also failed to demonstrate benefit to patients who received r-tPA in a 3- to 5-hour window.
Numerous studies have subsequently demonstrated the efficacy of intravenous r-tPA when given within the 3-hour window.10–12 Although the use of IV r-tPA was initially controversial, it has now been endorsed as class IA level of evidence by the major national guidelines development organizations.13 Pooled results from the six major randomized, placebo-controlled IV r-tPA stroke trials8,9,14 (ATLANTIS I & II, ECASS I & II, and NINDS I & II) included 2775 patients who were treated with IV r-tPA or a placebo. Treatment up to 3 hours after symptom onset benefitted patients, and findings suggested a benefit in treatment beyond 3 hours in certain subsets of patients. The ECASS III trial demonstrated the benefit of IV r-tPA in the 3.5- to 4-hour window in a more select group of patients.15
Recanalization rates, which are related to the diameter of the affected vessel, range from 20% for the ICA to 50% for occlusion of a distal branch of the MCA.16 Ischemic stroke related to large vessel (i.e., >2 mm) occlusion carries a high risk of mortality (53% to 92%) and severe morbidity.17,18 The presence of a hyperdense MCA sign on CT in the presence of a moderate stroke (National Institute of Health Stroke Scale [NIHSS] stroke score of >10) portends a less favorable therapeutic outcome with IV r-tPA. This finding suggests that large proximal clots are resistant to IV r-tPA.19 Endovascular therapies for acute stroke, particularly those related to large vessel occlusion, may overcome some limitations of systemic thrombolysis by providing superior recanalization rates in large vessel occlusions, reducing the incidence of intracranial hemorrhage, and expanding the therapeutic window.
Recanalization strategies
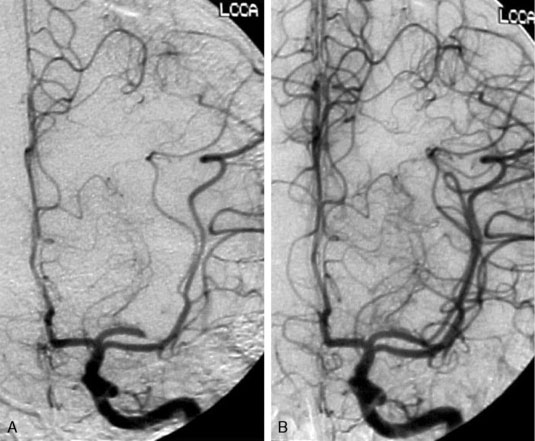
Recanalization aims to restore antegrade blood flow by removing or dissolving occlusive thrombus, thereby allowing reperfusion of the ischemic penumbra, the functionally impaired but still viable brain tissue. Endovascular techniques for recanalization include mechanical thrombectomy, chemical thrombolysis, stenting (either temporary or permanent), or a combination thereof (Figure 20–1).
The rationale for restoring antegrade flow as rapidly as possible after stroke is intuitive. The aphorism “time is brain” accurately reflects the ephemeral nature of the ischemic penumbra and underscores the importance of rapid restoration of blood flow. The ultimate outcome of stroke is not solely dependent on the time of occlusion of the target vessel. It also depends on a number of factors and their complex degree of interactions. These factors include the site of arterial occlusion, the extent of the core of infarction, the size of the ischemic penumbra, the quality and quantity of collateral arterial supply, and the integrity of the blood-brain barrier (BBB). However, on an epidemiological level, a number of studies have documented the correlation between radiographic recanalization and improved clinical outcome as well as the critical role of rapid restoration of flow.20
Intra-Arterial Thrombolysis
Intra-arterial recanalization is occasionally used as a primary treatment modality in patients in whom IV r-tPA is contraindicated or patients who fail to demonstrate clinical improvement after administration of IV r-tPA. Primary intra-arterial thrombolysis can benefit clinical outcomes of patients who receive treatment 3 to 6 hours after symptom onset.21,22 Compared to systemic IV thrombolysis, intra-arterial thrombolysis offers several theoretical advantages. Coaxial microcatheter techniques allow superselective access to the occluded vessel, thereby allowing infusion of thrombolytic agents directly into the thrombus. By infusing thrombolytic agents directly at the site of the thrombus, a smaller dose of fibrinolytic agent can result in more complete recanalization compared to systemic administration. Theoretically, rates of complications from systemic fibrinolysis such as ICH should be reduced.
Merci Retrieval System
The Merci Retrieval System was approved by the FDA in 2004 for the treatment of acute stroke due to thromboembolic occlusion of the intracranial vertebral artery, basilar artery, intracranial ICA, or M1 division of the MCA. The Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial was a prospective, nonrandomized, single-arm, multicenter trial that investigated the use of a novel mechanical thrombectomy device in patients with occluded large intracranial vessels within 8 hours of the onset of stroke symptoms.23 The primary endpoint of the trial was radiographic recanalization of the target vessel with a low rate of serious adverse events. The trial enrolled 151 patients and demonstrated recanalization rates of 48% with a complication rate of 7.1%. Morbidity and mortality rates were lower in patients who demonstrated radiographic recanalization.
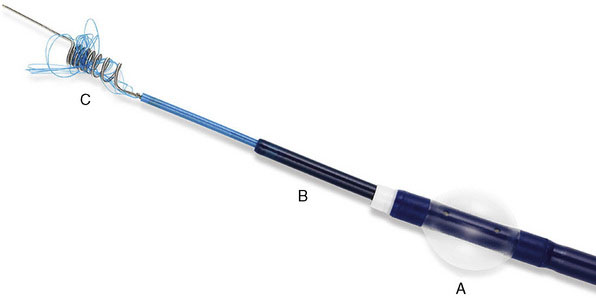
The Merci Retrieval System consists of the Merci Retriever, the Merci Balloon Guide Catheter (BGC), and the Merci microcatheter through which the actual retrieval device is deployed (Figure 20–2). The BGC is a 9-French catheter with a large 2.1-mm lumen and a balloon located at the distal tip. The Merci Retriever itself consists of a tapered wire with five helical loops of decreasing diameter (from 2.8 to 1.1 mm) at the distal end. The loops are constructed from memory-shaped nitinol (nickel titanium) and therefore exploit the superelastic properties of this alloy. Once the microcatheter is advanced distal to the clot, the Merci Retriever is advanced through the microcatheter in its straight configuration. Once deployed, the retriever reforms into its pre-established helical shape thereby ensnaring the thrombus. During this procedure, the balloon at the tip of the BGC (usually located in the common carotid artery or ICA) is inflated to minimize distal flow. A complete description of the technique can be found in the original description of the MERCI Phase I Study.24
On the basis of the results of the MERCI trial, the Merci Retrieval System was approved by the FDA for use in the treatment of acute stroke up to 8 hours after symptom onset. The approval of this device without a randomized trial has drawn some criticism, most notably due to the apparent increase in deaths associated with its use compared to the Prolyse in Acute Cerebral Thromboembolism (PROACT II—an earlier study evaluating the use of intra-arterial prourokinase) from 27% to 44%.25,26 However, the MERCI investigators argued that the presence of a higher percentage of distal ICA and basilar artery occlusions may have biased their results toward worse outcomes. The discrepancies in clinical outcomes underscore the need to accurately identify which patients are most likely to benefit from aggressive intervention.
Penumbra System
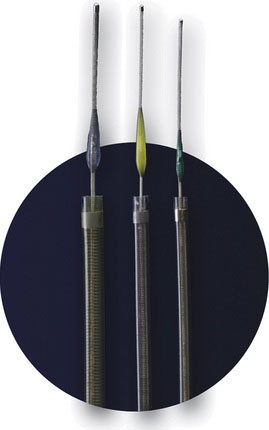
The PS is composed of three main components: a reperfusion catheter, a separator, and a thrombus removal ring (Figure 20–3). For aspiration, the reperfusion catheter is used coaxially with the separator while attached to an aspiration (suction) source to separate the thrombus from the vessel wall. If residual thrombus is still present after revascularization with aspiration, the thrombus removal ring can be used to directly engage and remove the residual thrombus. A complete description of the device and its use can be found in the original trial.27
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree