Fig. 1
MERCI Retriever. L5 device (above) and X6 device (below) engaging the clot (Courtesy of Concentric Medical, Inc.)
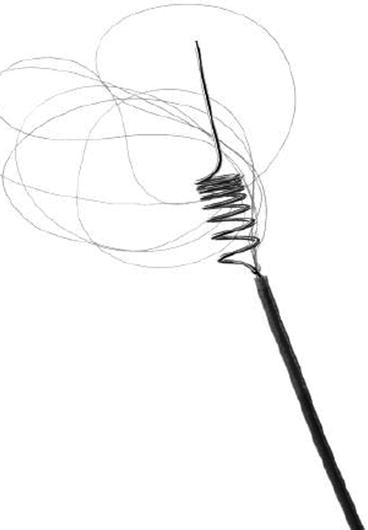
Fig. 2
MERCI Retriever – the latest V-series (With permission from Concentric Medical)
The efficacy of the device was tested in the MERCI phase I trial [23]. Part I included 30 patients attending seven centers in the USA. The main inclusion criteria were NIHSS score ≥10, endovascular treatment performed within 8 h of symptom onset, contraindication to IV tPA, no findings of large hypodensity on CT scan, and angiography-confirmed occlusion of a major cerebral artery. Safety was defined by the absence of vascular injury or sICH. Efficacy was assessed by recanalization rate and clinical outcome at 1 month. Significant recovery was defined as an mRS score of ≤2 at 30 days in patients with a baseline NIHSS score of 10–20 or an mRS score of ≤3 at 30 days in patients with a baseline NIHSS score of >20. The device was successfully deployed in 28 patients. Recanalization (TIMI 2 or 3) was achieved in 43 % of patients with mechanical embolectomy only, and in 64 % of patients with additional IA tPA. There was one procedure-related technical complication of no clinical consequence. Intracranial hemorrhage occurred in 12 patients, asymptomatic in all of them. At 1 month, 8 of 9 revascularized patients and 0 of 10 nonrevascularized patients achieved significant recovery.
Part 2 of the MERCI trial included 151 patients attending 26 centers in the USA, all with an INR of >3.0 [82]. The device was deployed in 141 patients. The recanalization rate was 53.5 %, and the favorable outcome rate (mRS ≤2 at 90 days) was 25 % [86]. The system received Food Drug Administration (FDA) approval in August 2005 [7].
The Multi-MERCI was an international multicenter single-arm trial with three objectives: to gain greater experience with the first-generation MERCI retrieval devices (X5 and X6) in patients ineligible for IV tPA; to investigate the safety and technical efficacy of the MERCI clot retriever in patients after failed IV tPA; and to collect safety and technical efficacy data on the second-generation L5 MERCI retriever [37, 81]. Inclusion criteria and techniques were otherwise similar to the MERCI I trial. The recanalization rate was 57.3 % with the L5 retriever alone (Fig. 2), and 69.5 % after adjunctive IA therapy based on angiography findings (n = 131). There was a 27 % absolute difference in mortality between patients who were or were not successfully recanalized.
Whereas the MERCI and Multi-MERCI trials were designed to study the MERCI embolectomy device in a trial context, with predetermined inclusion/exclusion criteria, the MERCI Registry study, initiated in June 2007, was an open-label prospective 36-center assessment of postmarketing real-world use of the MERCI retriever system in the interventional treatment of acute stroke. In 2010, an interim analysis of the registry period between June 2007 and December 2009 was performed (unpublished data) [36]. Of the 814 patients originally enrolled, procedural data were collected for 771 of whom 625 were included in the final cohort. (The others were lost to follow-up, missing data, or excluded because of a baseline mRS score of ≥2.) The findings were compared with the pooled MERCI/Multi-MERCI cohort of 305 patients. The MERCI Registry patients had a significantly lower baseline NIHSS score than the MERCI/Multi-MERCI patients (17.9 vs. 19.6, p < 0.0001), with no significant difference in comorbidities or medical history. Compared to the MERCI/Multi-MERCI cohort, the MERCI Registry cohort had a significantly longer onset-to-puncture time (5.72 ± 3.28 h vs. 4.35 ± 1.76 h, p < 0.001), lower percentage of patients who arrived less than 3 h from symptom onset (12.2 % vs. 20.8 %, p = 0.0008), and higher percentage of patients treated beyond 8 h (14.1 % vs. 1.3 %, p < 0.001). There was no between-group difference in puncture-to-recanalization time (MERCI Registry, 1.92 h; MERCI/Multi-MERCI, 1.89 h) or occlusion site (ICA 31 %, MCA-M1 49–52 %; vertebrobasilar circulation 8.2–9.2 %). The MERCI Registry group had a significantly higher rate of adjuvant pharmacological thrombolysis (IV lytics 27.8 % vs. 15.7 %, p < 0.0001; IA lytics 46.5 % vs. 31.8 %, p < 0.0001); 6.9 % received glycoprotein IIb/IIIa antagonists. Good revascularization, defined by the Thrombolysis In Cerebral Ischemia (TICI) IIb-III, was documented in 52.2 % of patients, with the lowest favorable recanalization rate in the ICA (45.6 %) and the highest in the vertebrobasilar circulation (64.7 %). The overall recanalization rate was 77 % compared to 64.6 % in the MERCI/Multi-MERCI cohort (p < 0.0001). There was no significant difference between the groups in favorable day-90 mRS score (≤2) or day-90 mortality (29–32 % vs. 34–38 %), or in rates of sICH, defined as a decrease of more than 4 points on the NIHSS (6.2 % vs. 8.8 %). The following results were found on multivariate analysis to identify predictors of good clinical outcome (mRS score ≤2 on day 90): baseline NIHSS score, OR 0.86 (p < 0.0001); age, OR 0.96 (p < 0.0001); revascularization, OR 2.97 (p < 0.0001); intubated state during the procedure, OR 0.43 (p = 0.0004).
It should be noted that the MERCI Registry study had several important limitations: nonrandomized cohort, nonmandatory consecutive enrollment, self-reported data, lack of a control group precluding comparisons with standard medical therapy, and lack of a core laboratory review. Nevertheless, it offers the largest prospective collection of data on mechanical embolectomy. In addition, using the registry, subsets previously too small for analysis could be analyzed, such as effect of intubation on outcome and time windows of treatment and procedure duration. The authors concluded that the real-world unconstrained experience with the MERCI embolectomy device for AIS yields similar clinical outcomes, safety data, and recanalization rates to those observed in previous trial studies. Successful recanalization was associated with higher rates of good clinical outcome [36].
Phenox Clot Retriever (pCR)
The pCR (Phenox GmbH, Bochum, Germany) is a more recent thrombectomy device designed specifically for intracranial clot removal. It has been available for use in Europe since 2006 [8]. The device is comprised of a dense array of soft perpendicular-oriented nylon fibers that gradually increase in length and attach to a flexible nitinol/platinum alloy compound core wire (Fig. 3). The fibers are resistant to unraveling and offer a high surface area for thrombus retrieval. The wire also carries proximal and distal visible X-ray markers for radio-opacity, facilitating handling and safety. The flexible design makes it possible for the surgeon to use two devices at once for bifurcations or larger vessels (kissing technique), and the microfilaments make it possible to lessen distal emboli owing to the filter effect. The device comes in three sizes measuring from 1 to 3 mm proximally and from 2 to 5 mm distally, for use in target vessels of ≥1, ≥, and ≥3 mm in diameter, respectively. It is shaped onto a 0.010-in. microguidewire and can be deployed using a standard microcatheter (0.021 or 0.027 in.). Animal experiments demonstrated that the device can be used for atraumatic retrieval of clots [65].


Fig. 3
Phenox clot retriever (pCR) (Courtesy of Phenox GmbH)
Another form of the pCR, known as the BONnet Intracranial flow Restoration Device (Fig. 4), consists of a nitinol braiding that self-expands into a cell-like structure to entrap the thrombus. Nylon filaments run through the braiding to provide additional surface area to keep the thrombus in place. Because of the braided design, the length of the device can be adapted to the vessel diameter [65]. The BONnet is positioned through a 0.021-in. microcatheter and deployed by pulling back on the microcatheter. After deployment, both the BONnet and microcatheter are slowly withdrawn under continuous aspiration into a guiding or aspiration catheter.
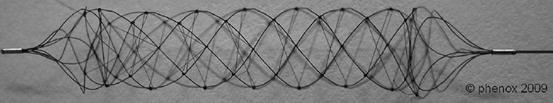

Fig. 4
Phenox clot retriever, BONnet device (Courtesy of Phenox GmbH)
Only a few small clinical reports on the use of pCRs for AIS are available [34, 51, 52]. The first, published in 2006, described two successive patients who were ineligible for IV/IA thrombolysis. Endovascular recanalization was successfully achieved in both, with complete neurological recovery and no adverse events [34]. The second study, presented at the International Stroke Conference in 2008 [52], reported a subset analysis of 60 vascular territory applications of the pCR in 55 patients treated in three centers in Europe (Bonn, Munich, and Stuttgart) in 2007. Most target vessels had a diameter of ≤2 mm. The majority were reached and recanalized without difficulty. A total of 85 % of occluded vessels were recanalized with the pCR, yielding a 68 % overall recanalization rate with distal reperfusion. When the device was combined with IAT, the recanalization rate rose to almost 80 % (30/38). There were no device-related adverse events. However, three attempts failed because the device and/or microcatheter could not be deployed. A third small series of three children with AIS included one treated with the pCR for occlusion in the BA [28]. Partial recanalization was achieved, with no adverse effects.
Like for the MERCI retriever, randomized controlled trials of the pCR are needed to validate its use in clinical practice. The pCR holds promise as a useful supplement to the repertoire of currently available devices for endovascular thrombectomy [8].
Catch Device
The Catch (Balt Extrusion, Montmorency, France) is an intracerebral thromboembolectomy device developed in 2005 [6, 8]. It is comprised of a self-expanding nitinol braided basket and a Vasco + 21 microcatheter equipped with a microguidewire (Fig. 5). The nitinol basket has a maximum diameter of 4 mm, which is suitable for clot retrieval in arteries up to 5 mm in diameter. A 6-Fr guide-catheter is recommended for use in conjunction with the device. The manufacturer has recently developed a mechanical thrombectomy kit consisting of a Balt Corail 8F+LT50 guide-catheter with occlusion balloon and distal extension for aspiration, a Balt Vasco+35ASPI microcatheter for proximal aspiration, and the Balt basket-like Catch device for clot retrieval. The Catch is deployed distal to the thrombus by retracting the microcatheter. The whole device is pulled back to withdraw the clot down to the guide-catheter, thereby reducing the risk of clot fragmentation. If further attempts to retrieve the clot are required, the resheathing hub included in the package may be used. Two in vivo animal studies have been conducted in Switzerland to evaluate the efficacy and potential complications of the Catch compared with the MERCI retriever [8, 11]. Both devices were used in 10 vessel occlusions: 4 in the ICA and 6 in the lingual artery (a branch of the external carotid artery). Seventy percent of the occlusions were successfully recanalized (TIMI grade 2–3) with the Catch device and 90 % with the MERCI retriever. The MERCI retriever was far superior in achieving recanalization at the first attempt (OR 21, 95 % Cl 1.78–248.11), such that significantly more attempts were made with the Catch device (n = 14; P = 0.02). Recanalization also took more time with the Catch device (average 20 min vs. 13 min). The Catch device caused more thrombus fragmentation during retrieval (OR 15.6. 95 % Cl 1.73–140.84) and more distal thromboembolic events. No dissections or perforations were reported, although the incidence of vasospasm was similarly high for both systems (average ate = 3). The authors concluded that both the Catch device and the MERCI retriever are effective for mechanical thrombectomy in patients with AIS, but the MERCI retriever is better. To account for the problem of loss of thrombus material, although not explicitly recommended by the manufacturer, the authors suggested that the Catch device be used with balloon occlusion and aspiration.


Fig. 5
Basket-like Catch device. Once deployed, the Catch device cannot be pulled back into the microcatheter (arrow) (Courtesy of BALT Extrusion)
Comparative Studies
A comparative in vitro study [8, 51] evaluated the effectiveness of five mechanical thrombectomy devices in thrombus retrieval and the risk of distal embolization for each: Catch (Balt), MERCI (Concentric), In Time and Attracter (Boston Scientific), and pCR (Phenox). Distal dislocation and retrieval of the thrombus was achieved with all devices at the first passage, although with markedly different rates of thrombus fragmentation and distal embolization. The MERCI, Catch, and pCR devices successfully removed the entire clot from the test tube, but the In Time and Attracter devices removed only part of the clot.
Despite encouraging results from the few studies that have been conducted thus far, it is clear that further research, particularly in the form of randomized trials, may provide compelling evidence as to the clinical effectiveness of endovascular treatment with the Catch device [8].
Proximal Clot Aspiration
Some thromboembolectomy devices are designed for use from the proximal to the distal part of the clot. Most of then used aspiration methods (Penumbra Device) or ultrasound (EKOS system). Since we already mentioned the EKOS on the IMS studies discussion, we complete the information of this group with the penumbra device.
Penumbra Aspiration System
The Penumbra Aspiration system (Penumbra Inc., Alameda, CA) is designed to remove intracranial occlusions by debulking, aspiration, and extraction [24]. It is comprised of an aspiration platform containing multiple devices that are size matched to the specific neurovascular anatomy (Fig. 6). A 2.8–5 F reperfusion catheter is connected to the aspiration pump, generating a suction force of −700 mmHg. Because the device works on the proximal surface of the occlusion, it optimizes safety and eliminates the need for navigation beyond the clot. Early clinical experience suggests that the Penumbra can be applied for successful revascularization in patients with acute stroke [24]. In an early study, Grunwald et al. [28] used the Penumbra to treat two of three patients aged 7–16 years with AIS; the remaining child was treated with the pCR. Partial recanalization was achieved with no adverse events. This prompted a larger, prospective, single-arm trial by the same group [29] including 23 patients with angiographically verified occlusion of a treatable intracranial vessel who presented within 8 h of symptom onset. Twenty-one target vessels in 20 patients were treated with the Penumbra system. All were revascularized to TIMI grade 2 or 3 (100 % success rate). At the 30-day follow-up, 45 % of patients showed an increase of 4 or more points on the NIHSS or an mRS score of ≤2. The mortality rate was 45 %, which was considered good in this cohort in which 70 % of patients had a baseline NIHSS score of >20 or BA occlusion [9]. The Penumbra was approved by the FDA in 2008.
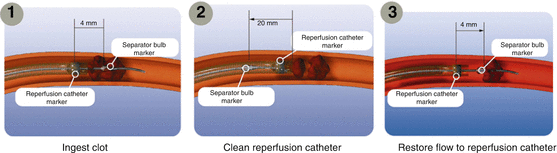

Fig. 6
Penumbra Aspiration System. During manipulation, the separator should completely exit the catheter and be withdrawn completely into the catheter (Courtesy of Penumbra Inc.)
The same year, Bose et al. [9], from the University of Saarland, reported the results of a 3-year prospective, nonrandomized, single-center controlled study of the clinical and functional outcome of 29 patients with stroke in the posterior circulation, including acute occlusions of the distal ICA (T-lesion), MCA–M1, or BA. Mechanical thrombectomy with the Penumbra was applied in patients who were ineligible for IV tpA or failed IV tPA administered within 3 h of symptom onset. Mean baseline NIHSS score was 20 for the whole cohort and 33.5 in the patients with BA strokes. Complete revascularization (TIMI 3) was achieved in 72.4 % of patients, and partial revascularization (TIMI 2) in 13.8 %. Revascularization failed in 13.8 %. The subgroup with BA strokes had a partial revascularization rate of 83.3 %. The NIHSS score improved by 4 points or more in 65.5 % of the cohort, and an mRS score of ≤2 was documented in 37.9 %. There were no device-related adverse events. Seven percent of patients had sICH [9].
Results of the Penumbra Pivotal Stroke Trial [71] were presented at the International Stroke Conference in February 2008 and published shortly thereafter. A prospective, multicenter, single-arm, nonrandomized design was used. The sample consisted of 125 patients with neurological deficits (NIHSS ≥8) at presentation and angiographically proven occlusion (TIMI 0 or 1) of a treatable large intracranial vessel (total, 125 target vessels). Patients were either ineligible for or refractory to tPA therapy or presented beyond the 3-h window (between 3 and 8 h of symptom onset). At 90 days after treatment, revascularization was successful (TIMI 2–3) in 81.6 % of treated vessels. Eighteen intraprocedural events were reported in 16 patients (12.8 %); in 3 patients (2.4 %), the events were considered serious. Intracranial hemorrhage was detected on 24-h CT scan in 35 patients (28 %), of whom 14 (11.2 %) were symptomatic. All-cause mortality was 32.8 % at 90 days, with 25 % of patients achieving an mRS score of ≤2. The authors concluded that the Penumbra is safe and effective for revascularization in patients with AIS secondary to large-vessel occlusive disease if administered within 8 h of symptom onset. Although the primary endpoint was met, this study was criticized for the low rate of 90-day functional independence. Goyal et al. [25] attempted to explain the enigma of the high revascularization rate on the one hand and lower-than-expected good-outcome rate on the other. Analysis of the pretreatment CT scans revealed that an ASPECTS score of >7 (small infarct) at baseline was associated with a higher likelihood of achieving functional independence after revascularization than a score of ≤7 (50 % vs. 15 %; p = 0.0001), and a total of 64 % of patients in the trial presented with large infarcts. This finding, along with other emerging data [95], supports the use of advanced imaging selection to guide the use of IA therapy.
Thereafter, 7 international centers published their collective post-marketing experience with the Penumbra Aspiration System in a retrospective review of 157 consecutive patients (Penumbra POST) [86]. Mean patient age at baseline was 65 years, and mean NIHSS score, 16. The rate of MCA occlusions (58 %) was lower than in the Pivotal trial, and the rate of vertebral artery occlusions (20 %) was higher, but neither of these differences was statistically significant (p < 0.05). Revascularization was documented in 87 % of the treated vessels: to TIMI 2 in 54 % and to TIMI 3 in 33 %, compared to 82 % in the Pivotal trial. In addition, 32 % of patients received adjunctive IA lytic treatment during the procedure, 18.5 % received IV lytic treatment prior to the procedure (refractory to tPA), and 23 % received both. Although the IA tPA group tended to have a higher rate of sICH, adjunctive lytic therapy did not materially affect the revascularization rate or the clinical outcome. There were 9 serious procedural events (5.7 %): 2 dissections, 2 perforations, one access site hematoma, one peripheral hemorrhage, and one cardiac arrest. Three device malfunctions occurred, including 2 fractures of the 032 reperfusion catheter at ~40 cm from the tip, and one breakage of the 041 separator at ~6 mm from the distal end of the device. The breakage was due to a hard clot that required the operator to pull back on the separator with considerable force after penetrating the thrombus. The tip of the separator stayed within the thrombus and was not retrievable. None of these malfunctions led to a serious adverse event or patient death. The all-cause mortality rate was 20 %. Forty-one percent of patients had an mRS score of ≤2 at 90 days compared to 25 % in the Pivotal trial. Although the postmarketing Penumbra data were not significantly different from the Pivotal trial data, the proportion of patients with a good functional outcome was higher than expected. A review of the Penumbra POST trial database did not reveal a definitive reason for the observed difference in outcome between the studies. However, it raised the intriguing possibility that it was at least partly attributable to an inherent variability in patient stroke pathophysiology at presentation.
The 2011 Penumbra SPEED study [20] assessed the safety and efficiency of the 054 catheter, which was added to the existing 3 catheters of the Penumbra system in 2009. It features a larger, 0.064 in., proximal internal diameter for enhanced aspiration efficiency. The interim results (n = 73) yielded a median aspiration time of 18 min compared with 45 min for the original catheters used in the Penumbra Pivotal trial (p < 0.001). The time from puncture to final revascularization was 53.5 min in the SPEED study and 97 min in the Pivotal Trial. The 054 catheter was associated with a high revascularization rate (TIMI 2–3) of 92 % when used as frontline therapy, prior to any adjunctive treatment.
Endovascular Bypass: Stentrievers
The use of stent retrievers (stentrievers) in AIS is based on the rationale that temporary endovascular bypass allows for rapid flow restoration. During stent deployment, the thrombus is displaced and entrapped between the stent and the vessel wall, and a channel is created inside the thrombus. No anticoagulation is needed as the stent can be recaptured without subsequent antiaggregation [83]. The literature on stent-assisted recanalization in AIS has been accumulating in the last few years. The first report on the deployment of a stent across a thrombus in AIS appeared in 2006 [10]. The first stent used in this context was the Enterprise (Cordis, Miami Lakes, FL).
Solitaire Self-Expanding Stent
The Solitaire (ev3 Inc., Plymouth, MN) is a self-expanding stent initially designed for stent-assisted coiling in remodeling of brain aneurysms (Fig. 7). It is currently offered in two versions: aneurysm bridging (AB) and flow restoration (FR). The Solitaire is unique in that it can be fully deployed and then completely retrieved if not detached [35, 87] once recanalization is established. Although detachment of the device is not within the primary scope for the treatment of ischemic stroke, it is a viable option if thrombectomy fails and thrombus compression by the deployed stent restores flow in the target vessel [72]. Other advantages include a closed-cell design, high comparative radial force, high cell deformation resistance, and good vessel-wall adaptation. Furthermore, the device is applicable multiple times and can be used even in small peripheral vessel branches (e.g., M2 segments). The FR variant was CE-marked for mechanical thrombectomy in July 2009.
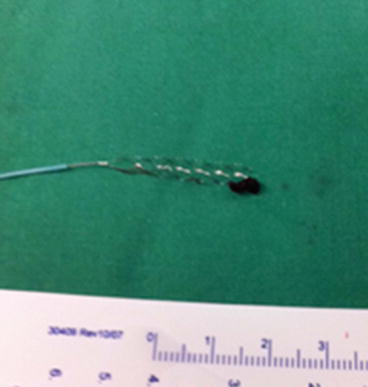

Fig. 7
Solitaire stentriever with a retrieved clot
The results of the first (pilot) study of the Solitaire AB stent in mechanical thrombectomy were published in 2010 in Stroke. Castaňo et al. [12] prospectively evaluated the intraprocedural and postprocedural findings in 20 patients with large artery occlusions of the anterior circulation attending a single tertiary center. All were refractory to or ineligible for IV tPA. Additional inclusion criteria were age ≤80 years, NIHSS score ≥8 (or lower if there was a fluctuating neurological deficit), <8 h between symptom onset and presentation, absence of large signs of ischemia (defined as an ASPECT score of <7 or magnetic resonance diffusion-weighted lesion in >50 % of the MCA territory), and proven occlusion of the anterior circulation on angiography. The treatable vessels included the terminus ICA, tandem proximal ICA/MCA, and MCA-M1 and -M2. The median NIHSS score was 19 (interquartile range, 15–23). An 8-Fr balloon guide-catheter was inserted via a femoral approach, so that the device was positioned in the proximal ICA. A heparinized saline solution was continuously perfused through the catheter during the procedure. With the balloon of the guide-catheter deflated, a 0.014-in. guide wire was advanced coaxially over a Rebar 18 microcatheter (ev3 Inc., Irvine, CA) within the occluded intracranial vessel and navigated distal to the clot. The microcatheter was then advanced over the wire through the clot, and the guide-wire was exchanged for the Solitaire AB embolectomy device. The device was advanced and deployed, with its distal portion placed a few millimeters distal to the clot. If the clot was shorter than the stent (20 mm), flow distal to the thrombus was restored immediately. The stent was maintained in place for 1 or 2 min, followed by inflation of the guiding catheter balloon to arrest the flow. The microcatheter and embolectomy device were gently aspirated simultaneously through the guiding catheter. Successful revascularization, defined as TICI 2b/3, was achieved in 90 % of treated vessels; flow was immediately restored in 80 %. The mean number of passes for maximal recanalization was 1.4, and the median (quartiles) time from groin puncture to recanalization was 50 min (range 38–71 min). In no case was adjuvant therapy required after deployment of the embolectomy device. There were no significant adverse procedural events. Distal embolization to the MCA territory occurred in two patients, but there were no cases of thrombus migration to a different territory. There were also no cases of arterial rupture or dissection. Two patients (10 %) had sICH: one fatal parenchymal hemorrhage, and one subarachnoid hemorrhage. Four patients (20 %) died during the 90-day follow-up period. Forty-five percent of patients showed a good functional outcome at 3 months (mRS ≤2). Age and NIHSS score were predictors of good outcome, in agreement with other reports on thrombectomy [82, 84]. The mean number of passes was lower than in the MERCI studies (2.9 passes), and the mean time from groin puncture to recanalization was shorter. This study suggested for the first time that the Solitaire AB device can rapidly, safely, and effectively retrieve clots from the MCA terminus and ICA within 8 h of symptom onset.
In other small case series, Miteff et al. [61] evaluated 26 consecutive patients, Nayak et al. [67] 7 patients, and Möhlenbruch et al. [63] 25 patients. Recanalization (TIMI grade ≥2) was achieved with Solitaire thrombectomy as monotherapy in 61.5, 100, and 88 % of patients, respectively. Corresponding rates of sICH were 7.7, 0, and 12 %. In the series of Miteff et al. [61], a favorable clinical outcome (mRS ≤2) was documented in 3 of 5 patients (79 %) with MCA occlusion, 6 of 11 patients (55 %) with ICA occlusion, and 2 of 10 (20 %) with BA occlusion; in the series of Nayak et al. [67], an improvement of more than 4 points on the NIHSS was documented in 5 patients (20 %), of whom 4 (57 %) had a 30-day mRS score of ≤2. No data on clinical outcome were available for the series of Möhlenbruch et al. [63]. Additional single-center experiences with the Solitaire for AIS are summarized in Table 1.
Table 1
Additional one-center experience published studies of the Solitaire stent-retriever device for acute ischemic stroke
Author, year (Ref.) | n | Onset-to-recanalization (mean, hours) | Baseline NIHSS (median) | Device + Adjuvantsa | Good recanalization (TIMI IIb/III) (%) | No. of attempts (mean) | mRS ≤ 2 (day 90) | Mortality | sICH |
|---|---|---|---|---|---|---|---|---|---|
Mpostsaris, 2012 [66] | 26 | 5.27 | 16 | 19/26 (73 %) | 69 | 1 | 10 (38 %) | 2 (7.7 %) | NA |
Cohen, 2012 [15] | 17 | 45 minb | 20 | – | 100 | 2 | 15c (88.2 %) | 1 (5.8 %) | 2 (11.8 %) |
Menon, 2012 [60] | 14 | 84 minb | NA | 9/14 (64.3 %) | 85.7 | 1–5 | 8 (57 %) | 2 (14.3 %) | 4d (28 %) |
Wehrschuetz, 2011 [94] | 11 | 5.6 | 16 | 11/11 (100 %) | 100 | 2.3 | 3 (30 %) | 1 (9 %) | 0 |
Stampfl, 2011 [83] | 18 | 4.8 | 21 (Mean) | 17/18 (94.4 %) | 88.8 | 2.5 | 6 (33 %) | 5 (2.7 %) | 3 (16 %) |
Roth, 2010 [77] | 22 | 4.6 | 19 | 13/22 (59 %) | 90.9 | 1 (40 %) | 11 (50 %) | 4 (18.1 %) | 2 (18 %) |
The two most comprehensive series of temporary endovascular bypass with the Solitaire were those of Liebig et al. [50] (50 patients and 53 occlusions) and Costalat et al. [17] in the Rescue, Combined Management and Stand-Alone Thrombectomy (RECOST) study (100 patients and 103 large-vessel occlusions). In the study of Liebig et al. [50], repeated stent manipulation was required in 64 % of patients; only six procedures (11 %) resulted in stent deployment. There were four adverse events, none device related. Immediate reperfusion (TICI ≥2a) was achieved in 73.6 % of procedures, extending the theory that stenting leads to relatively quick recanalization. The final reperfusion (TICI 2–3) rate was 86.6 %. The RECOST study used three different thrombectomy strategies based on time of symptom onset and location of vessel occlusion (MCA-M1 or -M2, 40 % of patients; ICA, 28 %; BA, 32 %). (1) Rescue therapy (24 % of cases) was applied in patients with MCA-M1 or -M2 occlusion who presented within 4.5 h of symptom onset and failed early IV tPA treatment (defined as NIHSS ≥8 60 min after IV fibrinolysis or neurological deficit significantly impacting quality of life). (2) Combined (bridging) therapy (56 % of cases) was used in patients with terminal ICA or tandem occlusions who presented within 4.5 h of symptom onset and patients with BA occlusions who presented within 24 h. No second neurological assessment was obtained before urgent thrombectomy, and IV fibrinolysis was continued during the endovascular procedure. (3) Stand-alone thrombectomy (20 % of cases) was used in patients who presented beyond the IV fibrinolysis therapeutic window (4.5–6 h for MCA and terminal ICA occlusions) and patients with well-accepted contraindications to IV fibrinolysis. Mean patient age was 67.6 years. All patients underwent clinical and magnetic resonance imaging (MRI) assessment. Mean NIHSS score at presentation was 14.7, and mean MRI ASPECT score was 6. Mean recanalization time from symptom onset was 377 min, with an overall recanalization rate (TICI 3) of 84 %. NIHSS score at discharge was 6.5; 60 % of patients had an NIHSS score of 0–1 or an improvement of >9 points. The symptomatic complication rate was 10 %. At 3 months, 54 % of patients had mRS score ≤2; the overall mortality rate was 12 %. The authors concluded that patient selection by MRI ASPECT score contributed to the low complication rates because it prevented futile and dangerous interventions [17].
The preliminary results of the ongoing Solitaire FR With the Intention For Thrombectomy (SWIFT) Study [80], the first to compare the Solitaire self-expanding stent with the MERCI retriever as initial therapy, were reported in 2012 at the International Stroke Conference. A multicenter, randomized, active comparator, noninferiority study design was used. Between January 2010 and December 2011, 58 patients were randomized to the Solitaire FR arm, and 55 to the MERCI arm. Primary endpoints were successful recanalization (TIMI 2 or 3 in all treated vessels), no sICH, and no rescue therapy (tPA, IIb/IIIa antagonists, etc.). Secondary endpoints were either good clinical outcome (mRS ≤2 or equal to the prestroke mRS if the prestroke mRS was higher than 2 or improvement by ≥10 in NIHSS score). A core lab blinded to the treatment assignment provided independent angiographic, CT, and MRI evaluations. Compared to the MERCI arm, the Solitaire FR arm had significantly higher rates of successful recanalization without sICH (core-lab-assessed) (60.7 % vs. 24.1 %, p = 0.0001), end-of-procedure successful recanalization (core-lab-assessed) (80.4 % vs. 57.4 %, p = 0.013), and good neurological outcome (mRS ≤2 or improvement in NIHSS score by ≥10) at 90 days (58.2 % vs. 33.3 %, p = 0.017), and a significantly lower 90-day mortality rate (17.2 % vs. 38.2 %, p = 0.02).
The sponsored multicenter prospective Solitaire FR Thrombectomy for Acute Revascularization (STAR) study [26] is the first “real-life” investigation of use of the Solitaire for acute stroke. The enrollment of 206 patients from 18 centers in Canada and Australia has just been completed. Inclusion criteria are presentation within 8 h of symptom onset and contraindications for IV tPA or failed IV tPA after bridging therapy or thrombectomy as initial treatment.
A search of PubMed until December 2012 using the combined keywords “Solitaire” and “stroke” yielded 43 publications of related devices to the Solitaire system. Early followers are discussed below, including the Trevo (Concentric Medical), the ReVive (Micrus Endovascular), and the Aperio (Acandis). Others include the IRIIS Plu and OptiCell (MindFrame, CA), the Pulse (Penumbra), and the pREset (Phenox) [72]. They differ in several features, but their intended clinical use is essentially similar.
Trevo Device
The Trevo (Concentric Medical Inc., Mountain View, CA) is designed for mechanical removal of occlusive thrombi in vessels ranging from 1.5 to 3.5 mm in diameter [59]. It measures 44 mm in length and 4 mm in diameter and has a hydrophilic coating to reduce friction during use. The device consists of a 180-cm long, proximal 0.018-in. core wire, with a 75-cm transition area at the proximal end and a closed-cell stent-like section at the distal end (Figs. 8 and 9). The proximal 10 mm are tapered for easy resheathing, and the distal 10 mm are tapered for smooth transition from the tip to the active area of the device. The distal tip is soft and floppy for safe and accurate deployment, and radiopaque to facilitate fluoroscopic visualization. The higher radial force across the active area allows for placement of the device in more distal and smaller vessels and more efficient clot retrieval and incorporation. A shaft marker indicates the proximity of the device to the microcatheter tip. By contrast to stents used to treat intracranial aneurysms (neck bridging) or intracranial atherosclerosis, in which the broader portion of the struts is in contact with the blood vessel wall in order to optimize coverage and wall apposition, in the Trevo device, the broader portion of the struts has an endoluminal orientation to optimize thrombus incorporation.
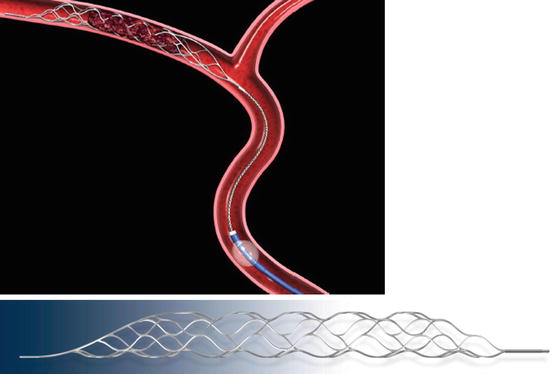

Figs. 8 and 9
Trevo stentriever (Courtesy of Concentric Medical Inc.)
Nogueira et al. [69] recently published their preclinical experience with the Trevo device in confirmatory animal models (2 swine, 1 canine) of arterial thrombo-occlusive disease induced by autologous thrombin-generated thrombi. Device performance was evaluated by angiographic response and clot incorporation by the device; high-resolution flat-panel three-dimensional CT was performed to further define the in vivo device-thrombus-vessel interaction. In addition, samples from vessels treated with 6 passes of the device were explanted for histopathological analysis. Sixteen clots of variable hardness and consistency were retrieved from the internal maxillary, lingual, and forelimb arteries (swine models) as well as from the external carotid and vertebral arteries (canine model). TIMI 2–3 reperfusion was achieved in all cases immediately after device deployment. One pass was required for 15 clots and 2 passes for the remaining clot. Histopathological analysis demonstrated severe disruption of the intima but no hemorrhage of the media or adventitia. The authors concluded that the Trevo device is highly effective at achieving immediate reperfusion of occluded arteries without causing any clinically significant disruption of vascular integrity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








