
Endovascular Treatment of Arteriovenous Malformations
Objectives: Upon completion of this chapter, the reader should recognize the indications for AVM embolization and the endovascular techniques used for safe and effective nidus occlusion.
Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians.
Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity.
The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp
* The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons.
The treatment of brain arteriovenous malformations (AVMs) is a complex task that requires close cooperation between the neurosurgeon, neuroradiologist, radiation therapist, and critical care specialist. Each individual case must have a clearly defined goal, based on its estimated natural history and risk of hemorrhage or other neurological sequelae and the risks of the therapeutic plan. The endovascular treatment of AVMs, with its benefits and risks, must be carefully integrated into the overall decision-making process. Since the first reported case in 1960, remarkable advances have been made in endovascular therapy that have improved both safety and efficacy. Advances in catheter design, particulate, liquid, coil-based embolic material, and imaging techniques have all contributed to this improved safety profile; however, the increased capability yields increased complexity and clinical responsibility. The goal of this chapter is to provide insight into the endovascular treatment of AVMs to hopefully provide order to this array of treatment options. To this end, we will discuss the indications, treatment strategies, techniques, and complications of endovascular therapy in the treatment of brain AVMs.
 Indications and Treatment Strategies
Indications and Treatment Strategies
Not all AVMs that can be treated necessarily need treatment. An understanding of the natural history of the disease and the risk of treatment is crucial when counseling patients about treatment options. The characteristics of the AVM, patient age, comorbidities, and psychosocial factors are all important to consider when developing a treatment plan. The patient and family’s expectations of treatment must be clearly understood, as they must understand the risks of the various stages of treatment and the entire treatment plan.
The prevalence of brain AVMs, based on autopsy studies, is ~0.15%,1,2 with the majority, 64 to 80%, of AVMs presenting before 40 years of age.3 Symptoms of AVMs include headache, seizures and epilepsy, focal neurological deficit, and intracranial hemorrhage. Headaches are quite common in patients with AVMs; they are the most common symptom leading to discovery of an AVM. Headaches and a variety of neurological deficits can occur that are unrelated to previous hemorrhage.2,3 These symptoms can be caused by decreased tissue perfusion, venous hypertension, and mass effect from dilated veins. Seizures can occur in from 28 to 67% of patients. Although the risk of bleeding is lower in patients with seizures than in patients with previous hemorrhage, it is higher than in those without seizures.
Hemorrhage is the most common single presenting symptom, occurring in over 50% of patients,4,5 with the estimated overall risk of hemorrhage from a brain AVM discovered incidentally or from evaluation of seizure or other nonhemorrhagic symptoms ranging from 2 to 4% per year.6–8 Each bleeding episode carries approximately a 10% mortality rate and a 30 to 50% morbidity rate due to persistent neurologic deficits,9 and the risk of rebleeding ranges from 6 to 18% in the first year but decreases to 2% per year after 10 years.7,10
Morphologic factors that increase the risk for AVM hemorrhage include intranidal aneurysms, restricted venous outflow, deep venous drainage, previous hemorrhage, small AVM size, diffuse AVM morphology, and higher feeding artery pressures.11 A more recent study has found that deep location and large size (>3 cm) were independent predictors of future hemorrhage.12 Also, hemorrhage at presentation did not necessarily predict higher subsequent hemorrhage risk. This study emphasized that the factors present at the time of initial hemorrhage are not necessarily the same factors that predict future hemorrhage. For example, small AVMs are thought to be more prone to hemorrhage, but this may be because small AVMs tend to present with hemorrhage, though they are less likely than large AVMs to cause other symptoms, such as seizures. It is beyond the scope of this chapter to discuss in detail the natural history of AVMs, but an important point to remember is that the natural history of many patients is benign, and observation alone may be a reasonable option.13
There are four main indications for endovascular embolization of brain AVMs: complete obliteration for cure, presurgical treatment, preradiosurgical treatment, and palliative flow reduction. Each of these indications carries a different decision algorithm and risk profile as the goals for each are quite distinct.
Complete occlusion of the AVM nidus by endovascular embolization alone is uncommon; reports of persistent cure rates in the literature vary from 5 to 32%.14,15 This relatively low rate reflects the varied approaches to AVM treatment at various centers, as many reports in the literature include the entire experience of AVM treatment, including embolization as an adjunct to surgery and radiosurgery rather than focusing on only those attempted for cure. The recanalization of AVMs angiographically “cured” by embolization has been reported; however, the permanency of occlusion is, at least partially, influenced by the embolization material used.16–18 Particulate embolization with polyvinyl alcohol (PVA) has been shown to be effective in controlling intraoperative bleeding; however, vessels embolized with PVA alone tend to recanalize after a few weeks.19–22 Although recanalization of “cured” AVMs with liquid embolic agents (Fig. 13-1), such as n-butyl cyanoacrylate (n– BCA), has also been reported, the incidence is much lower than with PVA.15,23,24 In comparison, complete surgical excision has nearly a 100% cure rate but carries a higher morbidity and mortality rate, and case reports of AVM recurrence after “complete” surgical excision do exist.25–27 Therefore, the use of endovascular embolization as the sole treatment modality for brain AVMs should only be considered in select cases with appropriate angio-architecture and perhaps be reserved for patients with relative contraindications to surgery or radiosurgery.
Presurgical embolization is the most widely used application for endovascular therapy of brain AVMs and is undertaken to reduce the surgical risks of uncontrollable hemorrhage and to improve surgical visualization. Clearly defined goals must be set, and open communication between the neurosurgeon and interventional neuroradiologist is critical in planning the course of therapy. Often, the easiest vessels to embolize are also the easiest to control during surgery and may not require embolization. Conversely, the more difficult vessels to embolize may be critical to successful surgical cure, and despite the increased risk, embolization is necessary in such situations. In considering presurgical embolization the combined risk of embolization and surgery must be less than that of the surgery performed without an embolization. Presurgical embolization is usually directed toward associated aneurysms, surgically inaccessible feeding pedicles, high-flow arteriovenous fistulae, and general reduction in flow within and the size of the nidus.
Aneurysms occur in 7 to 46% of patients with AVMs, and 40 to 75% of these aneurysms occur on feeding arteries28–30 probably related to vasculo-dynamic responses to increased flow. Whether or not to treat these aneurysms remains controversial,30–32 as studies indicate that the majority of feeding artery aneurysms will regress after removal of the AVM.28,29 In the largest review to date, Meisel et al30 evaluated 662 patients with AVMs and concluded that feeding artery aneurysms do not need to be treated prior to AVM treatment, and that after 3.5 years a 50% reduction in aneurysm size was noted in more than 50% of the cases. Persistent AVM occlusion with time was necessary to observe the size decrease, which raises the question as to the long-term effects of aneurysm shrinkage with partial AVM occlusion because the residual nidus will eventually recruit collaterals and pedicle flow will increase. Another study showed that following AVM treatment with less than 50% reduction in AVM size, no proximal feeding artery aneurysms had decreased in size, and two had enlarged and bled.32 The question remains as to the long-term hemorrhage risk of these residual aneurysms, those that have either remained stable and that have decreased in size. There is no question that if a patient presents with hemorrhage that is thought to be caused by a feedingartery aneurysm rather than the AVM, then the aneurysm should be treated and should be secured prior to attempting embolization of the AVM. Endovascular treatment of a ruptured feeding artery aneurysm can be a key adjunctive therapy, especially if it has a favorable configuration for endovascular embolization and is located anatomically too proximal to the AVM nidus to be reached by the neurosurgeon through a single craniotomy.
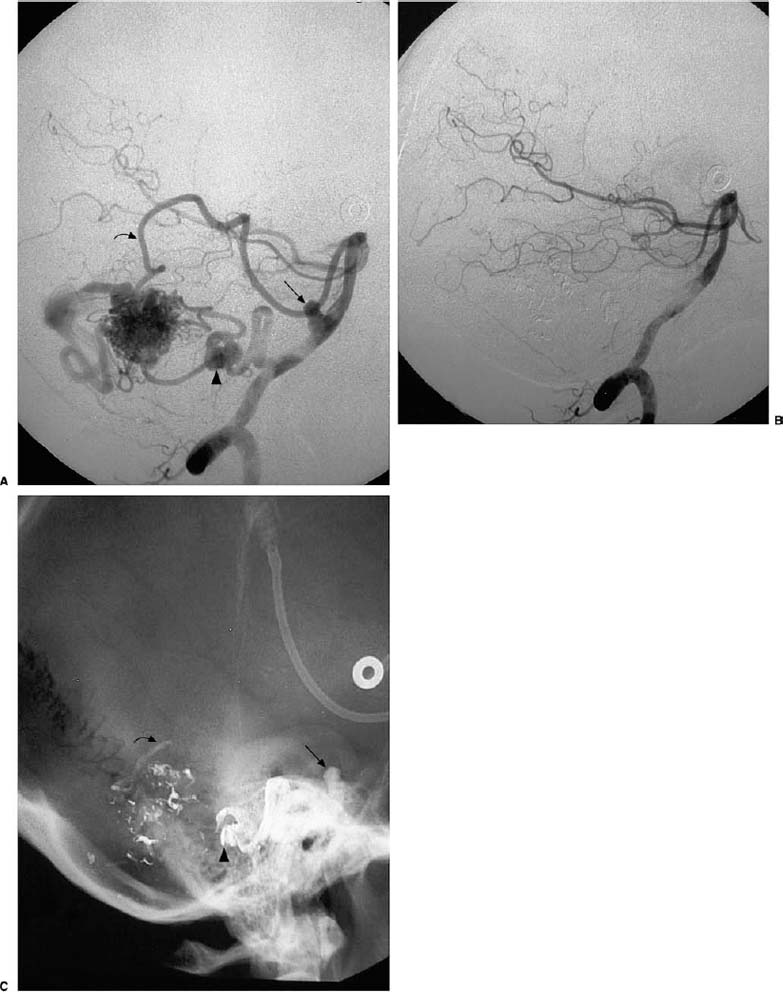
FIGURE 13-1 Angiographic obliterative embolization with n-butyl cyanoacrylate (n-BCA) in an arteriovenous malformation (AVM) with subarachnoid and intracerebellar hemorrhage. (A) Lateral right vertebral angiogram. Enlarged right posterior inferior cerebellar artery (PICA) with an origin aneurysm (arrow) and pedicle aneurysm (arrowhead) supplying a 2.5-cm AVM. The superior cerebellar artery (SCA; curved arrow) also provides supply. Based on the hemorrhage location the pedicle aneurysm was thought to have ruptured. Embolization of the PICA proximal to the aneurysm and the SCA just above the AVM resulted in complete obliteration of the AVM nidus and pedicle aneurysm on the postembolization angiogram (B) and radiograph of glue cast (C). Arrows and arrowhead correlate with same locations.
The occlusion of deep or surgically inaccessible feeding arteries is of critical benefit to a successful surgical cure. In fact, embolization of the superficial feeding arteries without treatment of the deep feeding arteries may lead to increased flow from these vessels and make the surgery much more difficult and dangerous than if no embolization was performed at all. Lenticulostriate, thalamoperforator, periventricular, choroidal, or deep posterior cerebral artery feeders are the most difficult to control at surgery and would be the best target vessels for endovascular embolization (Fig. 13-2). Unfortunately, these vessels are also often the most difficult to embolize and may carry too high of a collateral neurological risk due to the supply of normal structures en route to the AVM. Nonetheless, these vessels are often critical and require a coordinated effort between the endovascular and surgical specialist for the overall therapeutic plan.
On the other hand, embolization of surgically accessible superficial feeding pedicles is often performed with the goal of reduction of the nidal flow and overall size prior to surgery. Even though these pedicles can be clipped at surgery, the ability to embolize significant portions of the nidus via these pedicles can significantly reduce the surgical morbidity. Martin et al compared various aspects of preoperative embolization, including the degree of flow reduction and nidus occlusion, to the ease of surgical excision in 18 patients. They found that the only significant postembolization result that predicted easier, safer, and quicker surgical excision was nidus size reduction greater than 66%.31 This usually requires embolization of the majority of pedicles, surgically accessible or not.
Preoperative graded flow reduction to reduce normal perfusion pressure breakthrough is another useful application of endovascular therapy.31,33–35 Large AVMs with significant intranidal arteriovenous shunting have high-flow velocities and low pressure in the feeding arteries. The feeding artery branches supplying normal brain tissue adjacent to the AVM will also have low pressure within them. Local cerebral perfusion to normal brain tissue adjacent to the AVM is further decreased by relatively high shunt-induced venous pressures. The decreased perfusion pressure to the normal brain tissue adjacent to the AVM also results in decreased blood flow. This state of low perfusion pressure and low blood flow results is maximally vasodilated vessels that eventually lose their ability to autoregulate for changes in blood flow and pressure. A sudden decrease in high-flow arteriovenous shunting through the AVM, from either complete embolization or surgical resection, results in an abrupt increase in pressure in the feeding artery and its normal branches. Decreased flow through the AVM also decreases the high venous pressures that were present prior to treatment. These features combine to cause increased local arterial and brain perfusion pressure, and the lack of cerebrovascular compensation from the loss of autoregulation can result in cerebral edema and catastrophic hemorrhage, known as normal perfusion pressure breakthrough.
Numerous articles in the literature have reported edema and hemorrhage into surrounding brain tissue following abrupt hemodynamic changes precipitated by sudden removal of the high-flow arteriovenous shunting through the AVM.36–38 Certain angiographic characteristics of the AVM that might predispose to perfusion pressure breakthrough include large nidus size with high shunt flow; poor opacification of vessels to the normal surrounding brain; extensive collateral flow from other vascular territories (“sump effect”) including the external carotid arteries; and clinical symptoms of progressive or fluctuating, nonepileptogenic neurological deficits.38,39 Staged AVM embolization (Fig. 13-3) is a recommended method to gradually reduce the degree of arteriovenous shunting, thereby gradually increasing the pedicle pressure, reducing the venous outflow pressure, and improving the local cerebral autoregulatory function. Staged embolization usually consists of occlusion of a certain portion of the AVM, often a single vascular distribution, with second or third embolization sessions after a few days to a few weeks. The actual time between stages is controversial; however, most interventionists separate them by at least 1 week to reestablish compensatory autoregulation. Following the staged embolization, complete surgical resection of the AVM can proceed with a reduction in the risk of normal perfusion pressure breakthrough and its devastating sequelae.
Radiosurgery is an important alternative to surgical resection in the treatment of certain brain AVMs. Arteriovenous malformations located in areas of the brain that are surgically inaccessible due to the involvement of significantly eloquent tissue or are morphologically too dangerous for surgical resection are often considered for radiosurgery. This technique has up to an 80% cure rate with low morbidity and mortality in AVMs less than 3 cm in diameter; however, the efficacy and safety significantly decreases in AVMs greater than 3 cm and in AVMs with intranidal fistulas.24,40 One study suggested that embolization prior to radiosurgery may be beneficial to reduce AVM nidus size and therefore radiosurgical target volume—to eliminate high-risk AVM features, such as intranidal aneurysms, that might predispose to hemorrhage during the 2- to 3-year delay before the radiation-induced AVM obliteration occurs—and to eliminate high-flow intranidal fistulas, which may be refractory to radiation therapy.41 However, for small AVMs it is unclear whether or not embolization prior to radiosurgery shows any benefit compared with radiosurgery alone. Studies show that for small AVMs, the overall cure rates are similar for embolization prior to radiosurgery compared with radiosurgery alone.40,41 For medium-sized AVMs (3 to 6 cm) there may be some benefit to preradiosurgical embolization. The risks of preradiosurgical embolization for large AVMs (>6 cm) appear to outweigh the benefits. In fact, the benefits of radiosurgery in the treatment of large AVMs are highly suspect, and embolization has little effect upon this outcome. Attempts at nidus size reduction with embolization to decrease the size of the radiation focus have yielded limited results; although, as with embolization for obliterative cure, treatment with liquid agents in this setting is more effective than particles. Nonetheless, there does not appear to be any significant protective effect from hemorrhage during the latent period between radiosurgery and radiation-induced AVM obliteration from preradiosurgical embolization in these large lesions. In addition, recanalization of previously embolized portions of the AVM nidus can be a source of incomplete AVM obliteration following radiosurgery.24
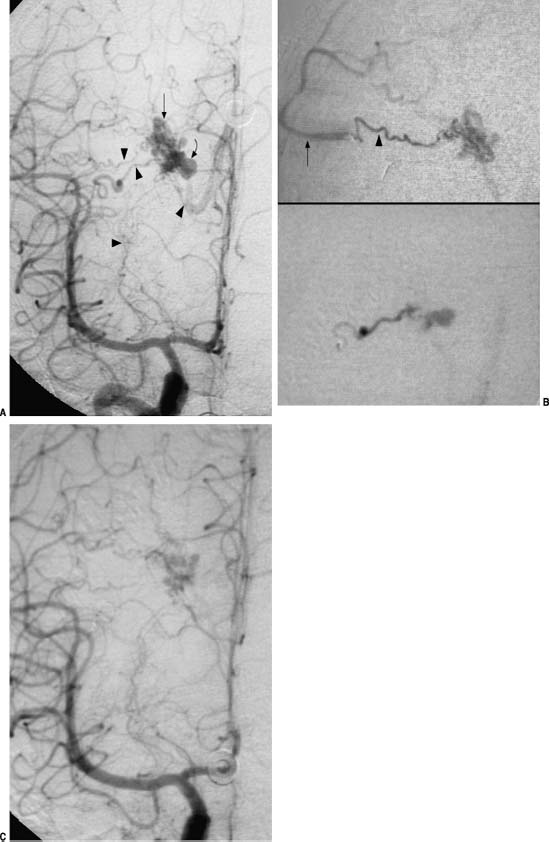
FIGURE 13-2 Embolization of deep perforating artery, arteriovenous fistula, and varix in an arteriovenous malformation (AVM) with intraventricular hemorrhage. (A) Anteroposterior (AP) right internal carotid angiogram. A small, deep AVM nidus (arrow) with varix (curved arrow), the most likely source of hemorrhage, is being supplied by (arrowheads, bottom to top) lenticulostriate, sylvian perforators, and a peripheral perforator. The lenticulostriate is small and has too many normal branches en route. The sylvian branches were approached, and on the AP super-selective angiograms (B) only the lower branch (arrowhead) was appropriately engaged; the upper branch resulted in reflux into the middle cerebral artery (MCA; arrow). This vessel was embolized with a wedged injection and occlusion of the lower supply and varix as shown on the postembolization angiogram (C). The surgeon was able to clip the lateral perforating vessels (arrowheads) prior to engaging the AVM, which had completely thrombosed at surgery the following day.



