Pregnancy
Puerperium
Infection related
Direct septic trauma
Cerebral abscess
Subdural empyema
Meningitis
Tuberculous meningitis
Otitis media
Orbital cellulitis
Tonsillitis
Dental infections
Stomatitis
Cellulitis
Septicemia
Pulmonary tuberculosis
Endocarditis
Measles
Hepatitis
Herpes simplex
Varicella zoster
Cytomegalovirus
HIV
Malaria
Trichinosis
Toxoplasmosis
Aspergillosis
Cryptococcosis
Hypercoagulable disorders
Protein C deficiency
Protein S deficiency
Antithrombin III deficiency
Factor V Leiden mutation
Prothrombin gene mutation
Homocystinemia/homocystinuria
Essential thrombocythemia
Primary polycythemia
Plasminogen deficiency
Tissue plasminogen deficiency
Elevated plasminogen activator inhibitor-1
Dysfibrinogenemia
Heparin-induced thrombocytopenia (HIT)
Increased factor VIIIc
Medication related
Oral contraceptive pills
Androgens
Antiestrogen therapy
Antineoplastic agents: cisplatin, l-asparaginase
Sildenafil
Carbamazepine
Malignancy
Squamous cell metabolic cervical cancer
Non-Hodgkin’s lymphoma
Bilateral glomus tumors
Colorectal cancer
Epidermoid carcinoma of tongue
Dysgerminoma
Ewing’s sarcoma
Allogenic transplant for acute lymphoblastic leukemia
Paraneoplastic syndrome
Meningioma
Rheumatologic disease
Behcet’s disease
Antiphospholipid antibody syndrome
Systemic lupus erythematosus
Wegener’s granulomatosis
Churg–Strauss syndrome
Other conditions
Nephrotic syndrome
Paroxysmal nocturnal hemoglobinuria
Iron deficiency anemia
Sickle cell anemia
Inflammatory bowel disease
Trauma
Lumbar puncture
Endocrine disorders: diabetes, thyroid disease
Renal allograft
Dehydration
Anemia
Prolonged airline flights
Occlusion of the cerebral veins causes localized edema and venous infarction. Microscopic examination shows enlarged, swollen veins, edema, ischemic neuronal damage, and petechial hemorrhages. These petechial hemorrhages can merge to become large hematomas. Both cytotoxic (cellular swelling in the setting of apoptosis) and vasogenic (blood-brain barrier breakdown and extravascular extravasation of fluid) edemas occur [9].
A second, less dramatic cause of clinical symptoms in CVT is increased intracranial pressure (ICP). This is generally caused by isolated or predominant dural sinus thrombosis. Thrombus in this location leads to poor CSF drainage and absorption by the arachnoid granulations. A combination of these two mechanisms is often present in the most severe cases [10].
Clinical Presentation
Consistent with the variations in the underlying pathophysiology, clinical presentations also vary significantly, depending of the type and extent of venous thrombosis. This variability makes diagnosis, at times, challenging. There are three large categories of clinical features [11]:
Headache with/without papilledema: headache is by far the most common symptom in CVT, occurring in 90 % of cases. It may occur suddenly, but more commonly is gradual in onset. The triad of headache, vomiting, and papilledema occurs in only 20–40 % of CVT patients, but is the most consistently identified clinical pattern.
Focal brain irritation/injury: focal neurologic signs, including sensory and motor deficits, aphasia, or hemianopia, develop in 40–60 % of patients with CVT. Seizures occur in about 40 % of patients. Seizures may be focal or generalized. Status epilepticus is one of the more damaging manifestations of CVT.
Obtundation/coma: found in 15–19 % of patients at presentation. These signs are usually seen in patients with extensive thrombosis of the deep venous system and bilateral thalamic involvement or with generalized seizures. This presentation can also be seen in patients with large unilateral lesions causing mass effect and herniation. Coma at presentation is the strongest predictor of poor outcome in CVT [10, 12].
Radiological Features
The most commonly utilized neuroimaging studies, non-contrast head CT and MRI, may be normal in CVT patients. Hyperdensity of the dural sinuses may be detected by the meticulous neuroimager but is only 25 % sensitive for this condition [13]. The famous “empty delta” sign is seen on contrast CT or MRI. An axial or coronal image through the torcular Herophili or superior sagittal sinus will show the triangular enhancement of the dural walls, but no contrast enhancement within the lumen due to clot. However, even this sign is only found in 30 % of CVT [14]. For this reason, venography is crucial to rule out CVT. MR venography and CT venography are both effective tools to image the dural sinuses, but are less sensitive for cortical vein and deep vein thrombosis. The sensitivity may be increased with catheter angiography, but must be accompanied by a high index of suspicion on the part of the angiographer. Catheter angiography also has the advantage of providing a dynamic assessment of venous flow/flow restriction [15] (Fig. 8.1).
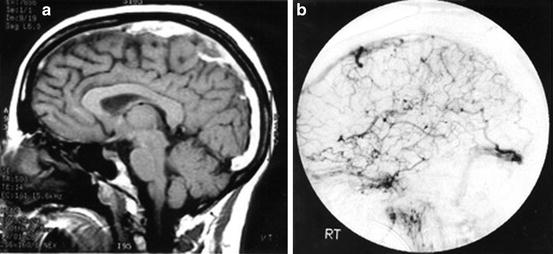

Fig. 8.1
(a) Gadolinium-enhanced sagittal MRI showing thrombus within the superior sagittal sinus (SSS). (b) Lateral projection cerebral angiogram confirming SSS thrombosis and demonstrating the collateral venous drainage patterns
Medical and Surgical Management
Once CVT has been diagnosed, the first line of therapy should include aggressive hydration and anticoagulation. The evidence base for anticoagulation in the setting of CVT is not deep, but the two published, prospective, randomized trials point to its benefit [16, 17]. A meta-analysis showed over 50 % reduction in death and disability compared to placebo [18]. It is important to emphasize that anticoagulation appears effective even in the setting of intracerebral hemorrhage (ICH).
Both unfractionated (UFH) and low molecular weight heparin (LMWH) have been used to achieve anticoagulation in the acute setting [16, 17]. UFH has the advantage of rapid normalization of coagulation parameters once the intravenous drip is turned off LMWH, on the other hand, maintains a more consistent therapeutic effect. In a mildly affected patient (i.e., headache and papilledema), LMWH may be relied upon. However, in the unstable or deteriorating patient, UFH should be utilized. This is especially true in the setting of ICH where hematoma expansion would necessitate prompt cessation of the anticoagulant effect. Although clinical trial evidence is lacking, it is common practice to transition from UFH or LMWH to oral anticoagulation once clinical stabilization/improvement is noted. The duration of oral agent use can be tailored to the particular patient. Typically, it is maintained until clot resolution is visualized on follow-up imaging (3–6 months). In patients with ongoing risk (e.g., a hypercoagulable condition), lifetime treatment may be required [19].
Seizure prophylaxis in CVT patients is somewhat controversial, but should be considered when significant cortical edema or hemorrhage has occurred [20]. Once seizures have occurred, however, initiation of an antiepileptic agent (AED) is universally recommended [21]. Status epilepticus is a not uncommon and particularly deadly manifestation of CVT and requires the use of intravenous agents. Fosphenytoin and levetiracetam are effective agents with good side effect profiles. It is reasonable to continue oral AED treatment for up to 1 year to prevent delayed seizure activity.
Surgical therapies are also effective and at times life saving in CVT. Extraventricular drain (EVD) placement is particularly effective in patients with a predominant involvement of the dural sinuses and impaired CSF drainage due to venous hypertension and obstruction of the arachnoid granulations by clot. In patients with significant preexisting parenchymal injury and/or a herniation syndrome, however, EVD placement is generally not helpful [22]. However, decompressive surgery (i.e., hemicraniectomy) does appear to be effective in preventing death under these dire circumstances. Such surgeries may also reduce long-term disability [23, 24].
Endovascular Treatment
Patient Selection
Due to the low incidence of CVT and high rate of favorable outcomes following medical therapy alone, endovascular therapy’s role has taken only incremental steps forward over time. Current indications include:
Progression of neurological deficits despite therapeutic anticoagulation: the failure of medical management in this setting has been defined using various criteria. A progression of neurological symptoms despite therapeutic anticoagulation is one common definition. Some authors feel that the neurological deterioration must be severe or even life threatening in nature to justify this approach. However, if neurological damage is allowed to occur, these patients may be too profoundly affected to respond successfully to recanalization [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








