1.$The diameter of the proximal part of the artery at its widest, non-tortuous, normal segment is chosen (first choice)
2.$If the proximal artery is diseased, the diameter of the distal portion of the artery at its widest, parallel, non-tortuous normal segment is substituted (second choice)
3.$For the internal carotid artery disease involving the pre-cavernous, cavernous, and postcavernous segments, the petrous carotid segment with parallel margins is measured at its widest, non-tortuous, normal portion
4.$If the entire petrous carotid is diseased, the most distal, parallel part of the extracranial internal carotid artery is substituted (second choice)
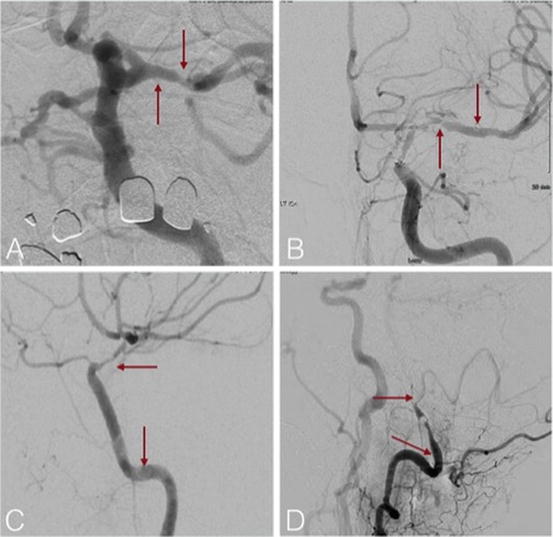
Fig. 5.1
Measurement of intracranial stenosis using the WASID method. (a) The diameter of the proximal part of the artery at its widest, non-tortuous, normal segment is chosen (first choice). (b) If the proximal artery is diseased, the diameter of the distal portion of the artery at its widest, parallel, non-tortuous normal segment is substituted (second choice). (c) For the internal carotid artery disease involving the pre-cavernous, cavernous, and postcavernous segments, the petrous carotid segment with parallel margins is measured at its widest, non-tortuous, normal portion. If the entire petrous carotid is diseased, the most distal, parallel part of the extracranial internal carotid artery is substituted (second choice)—not shown. (d) If the entire intracranial artery is diseased, the most distal, parallel, non-tortuous normal segment of the feeding artery is measured (third choice)
For noninvasive modalities of evaluating ICAD, a study assessing the accuracy of TCD and MRA compared with DSA showed the TCD and MRA to have good negative predictive values of 86–91 % but low positive predictive values of 36–59 % [15, 26]. Another study comparing CTA with MRA, using DSA as the reference standard, CTA was shown to have a higher sensitivity, specificity, and positive predictive value [29, 39]. The higher sensitivity and specificity of CTA have been observed specifically for stenosis which is 50 % or higher [30]. As far as the evaluation of small intracranial arteries, a study comparing multidetector CT (MDCT) angiography to DSA concluded that MDCT depicted ≥90 % of all examined small intracranial arteries compared to DSA, and the smallest arterial size reliably detected with CTA was 0.7 mm versus 0.4 mm for DSA.
Imaging of Vessel Wall
Vessel wall imaging can be achieved with high-resolution 3T MRI, intravascular ultrasound, and fat-suppressed T1-weighted MRI. High-resolution MRI can identify the thickness and pattern of protrusion [31, 32]. Intravascular ultrasound can be used for plaque components like calcium and lipid but is limited in its use due to its invasive nature [33]. Identification of recent intra-plaque hemorrhage and inflammation can be made with fat-suppressed T1-weighted MRI on which these plaques show increase in signal and enhancement after contrast injection [34, 35, 40, 41].
Collateral Assessment
The degree of collateral circulation is a powerful predictor of recurrent stroke in the setting of medical therapy for symptomatic ICAD [11, 42]. Impaired distal territory perfusion can be compensated for with good leptomeningeal collaterals. The ASITN collateral score (Table 5.2) is the most commonly used grading system [36, 43].
Table 5.2
ASITN collateral score grade description
0 = no collaterals visible to the ischemic site |
1 = slow collaterals to the periphery of the ischemic site with persistence of some of the defect |
2 = rapid collaterals to periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory |
3 = collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase |
4 = complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion |
Surgical Treatment of Intracranial Stenosis
Multiple surgical treatments such as direct and indirect bypass surgeries have been developed and used for over 40 years in treatment of intracranial stenosis. Most recently, the Carotid Occlusion Surgery Study (COSS) evaluated patients with ipsilateral ischemic events within 120 days in the setting of cervical carotid occlusion. Patients were randomized if they exhibited an increased oxygen extraction fraction and were randomized to medical treatment versus EC/IC (extracranial to intracranial) bypass surgery. Stroke and death at 30 days and 2-year ipsilateral stroke rates were not statistically different between the surgical and medical group (21 % versus 23 %) [37, 43–45]. Interestingly, the surgical group did experience decreased oxygen extraction fraction but no cognitive outcomes testing was conducted.
Prior to the COSS trial, the EC/IC bypass study showed no benefit of surgical bypass versus medical therapy for the reduction of overall ipsilateral major strokes or death. This trial included patients with both intracranial and extracranial stenotic and occlusive diseases [38, 46]. Surgical treatment used a direct bypass from the superficial temporal artery to the middle cerebral artery at one of the M2 branches. The medical arm was limited to single antiplatelet use (aspirin 325 mg, four times daily) and blood pressure reduction but no specific lifestyle modification regimen [38, 46–48]. Due to the results of these studies, EC/IC bypass is not generally recommended in the setting of ICAD.
Medical Treatment of Intracranial Atherosclerotic Stenosis
The medical treatment or ICAD has evolved over the years. The WASID trial was the first major trial to evaluate medical treatments and compared aspirin versus warfarin in patients with ICAD. The overall risk was similar in the warfarin and aspirin arms, with the primary endpoint of ischemic stroke, brain hemorrhage, or vascular death occurring in 22.1 % of patients assigned aspirin versus 21.8 % in the warfarin group. However, the warfarin cohort had significantly more adverse events defined as death, major hemorrhage, and myocardial infarction or sudden death [15, 49]. Next, the GEISCA study demonstrated that despite medical treatment, the 2-year rate of ischemic events in the territory of the stenotic artery was 38.2 % with the highest risk of stroke in clinically significant hemodynamic stenosis [39, 50]. Most recently, the Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) trial revealed much better outcomes with medical treatment. At 1 year, the event rate was 17.6 % in the stenting arm and 12.2 % in the medical management arm [2, 6, 51]. The medical arm of SAMMPRIS is now considered the standard of care for first-time symptomatic ICAD patients. The regimen was aspirin 325 mg and Plavix 75 mg daily for 3 months, followed by aspirin only. Additionally, patients were treated with a “statin” medication, blood pressure control, and were enrolled in a lifestyle modification program. Goal systolic blood pressure was less than 140 mmHg and LDL was less than 70 mg per deciliter (1.81 mmol/L). In addition to the above regimen, management of secondary risk factors [diabetes, elevated non-high-density lipoprotein (non-HDL) cholesterol levels, smoking, excess weight, and insufficient exercise] was included.
Endovascular Treatment of Intracranial Atherosclerotic Stenosis
Endovascular treatment of intracranial stenosis can be divided up into three possible treatments: balloon angioplasty alone (BA), balloon-mounted stenting (BMS), and self-expanding stent (SES) placement. Indications for each procedure (Table 5.3), outcomes from literature, and example case presentations are provided in the chapter.
Table 5.3
Possible indications and FDA indications for endovascular treatment
1.$Hemodynamic symptoms |
2.$Poor collaterals |
3.$Large mismatch on imaging with signs of collateral failure |
4.$Recurrent symptoms despite best medical therapy |
5.$FDA Wingspan use criteria: (1) age 22–80 years (2), two or more strokes despite aggressive medical management (3), most recent stroke occurring more than 7 days prior to planned intervention (4), 70–99 % stenosis due to atherosclerosis of the related intracranial artery, and (5) good recovery from previous stroke and modified Rankin score of ≤3 prior to intervention [65, 66] |
Intracranial Balloon Angioplasty Without Stenting
Stand-alone intracranial balloon angioplasty has been advocated by some over stenting, based on case series with low periprocedural complication rates [40, 41, 52]. Balloon angioplasty was first used in the coronary circulation. The goal of angioplasty is to reduce luminal stenosis and increase perfusion to downstream tissue. The proposed mechanisms include plaque redistribution and dilatation of the actual vessel diameter [40, 42, 53, 54]. This was initially described with percutaneous transluminal angioplasty (PTA) of the coronary arteries.
Initially there was some enthusiasm for the use of balloon angioplasty in the intracranial circulation with the first reported cases in the 1980s [40, 41, 43, 53–57]. There were cases of basilar followed by cavernous segment carotid and then middle cerebral artery stenosis treated with angioplasty [40, 43–45]. Unfortunately due to higher rates of complications, the use was limited until new techniques and technologies were developed [46, 56]. Dissections, emboli, and rupture were not uncommon with a complication rate of up to 50 % reported in one study [46–48, 53]. These complications were attributed to the large size of balloons and the rapid rate of inflation. Unlike coronary vessels, the intracranial circulation is surrounded by brain and cerebrospinal fluid in the subarachnoid space. A muscular myocardium as well as a pericardial sac surrounds the coronary arteries. A small rupture or dissection is usually not significant in the coronary circulation. On the other hand, a subarachnoid hemorrhage can be fatal with morbidity and mortality reported in up to 40–50 % of patients [49, 58–60]. Intraparenchymal hemorrhages also carry a high rate of morbidity and mortality [50, 61]. Dissections can cause ischemic strokes that can also cause a high morbidity. Stroke, largely ischemic, remains the number 1 cause of disability [52, 62]. With slow inflation over minutes as opposed to seconds and undersized balloons, complication rates have been reported as low as 5 % [40, 53, 54, 63] (see Fig. 5.2).

Fig. 5.2
(a, b) Patient with vertebral artery stenosis with recurrent strokes on optimal medical therapy before and after balloon angioplasty cerebral angiogram in AP projections, (c, d) patient with in-stent intimal hyperplasia before and after balloon angioplasty cerebral angiogram in AP projections
There have been multiple recent case reports of balloon angioplasty for intracranial stenosis with low complication rates and technical success rates to above 90 %. Defining technical success as less than 50 % stenosis (established by the Practice Guideline Committee of the Society of Neurointerventional Surgery) puts modern series at a technical success rate of 60 to above 90 % [3, 40, 41, 53–57, 64, 65]. The largest study by Marks et al. included 120 patients [3, 20, 40]. Some studies have suggested that restenosis and outcomes in balloon angioplasty without stenting versus stenting are similar as was demonstrated by a comparison paper of angioplasty alone versus stenting by Siddiq et al. [56, 66].
Balloon Types
There are several intracranial balloons that have been approved for use, but only one for the treatment of ICAD. These include the Scepter (MicroVention; Tustin, CA, USA), HyperForm (Covidien, Irvine, CA), and Transform (Stryker, Kalamazoo, Michigan, USA). These devices are designed for balloon-assisted coil embolization of aneurysms. The Gateway (Stryker, Kalamazoo, Michigan, USA) balloon is the only FDA-approved device for intracranial angioplasty. Coronary angioplasty balloons have been used off label to treat ICAD [51, 53].
Drug-Eluting Stents
Drug-eluting stents (DES) have been used off label in the intracranial circulation, and multiple reports have been published on their use. DES have been used in both the anterior and posterior circulation with periprocedural complication rates ranging from 0 to 25 % [3, 58–60]. A design limitation of DES is that most are balloon mounted and difficult to track in the intracranial circulation. Other criticisms include the need for dual antiplatelet therapy for 6 months or longer as suggested by some of the cardiac literature [61, 67]. There is some literature on newer DES that points to a lower incidence of delayed thrombotic events, but research is still ongoing [54, 62]. The lack of long-term follow-up in patients treated with DES has limited their acceptance. However, recent small case series (n = 11) with a mean follow-up period of 67 months has shown no patient with greater than 50 % restenosis [63, 68].
Balloon-Mounted Stents
Balloon-mounted stents (BMS) have also been used in ICAD with similar success as seen with balloon angioplasty alone. Most of the reported literature has used coronary BMS. The only published data on a dedicated intracranial BMS system is from the SYLLVIA trial that used the NeuroLink system. The difficulty with the current BMS systems is that they are stiff and, therefore, harder to track in the tortuous intracranial circulation. The SYLLVIA trial also showed a 35 % restenosis rate although 61 % were asymptomatic.
Self-Expanding Stents
Self-expanding, nitinol, stents have been the main of intracranial stenting ever since the FDA approval of the Wingspan Stent (Stryker, Kalamazoo, Michigan, USA). They have had high technical success rate ranging from the 98.8 to 96.7 % in the two large reported registries and 94.6 % (12/224) in the SAMMPARIS randomized control trial with seven patients having procedure aborted and five having only angioplasty for technical reasons [3, 56, 64, 65]. It has been argued that this system is more appropriate for use in the intracranial circulation due to its small outward radial force (<0.1 atm). It also does not need a balloon and can be delivered thru a microcatheter, making it more trackable. Despite these advantages, the SAMMPRIS trial, short- and long-term outcomes, revealed better outcomes in the rate of stroke and death in the medically treated group. At a median follow-up of 32.4 months, 34 (15 %) of 227 patients in the medical group and 52 (23 %) of 224 patients in the stenting group had a primary endpoint event [3, 20, 57] (Fig. 5.3).
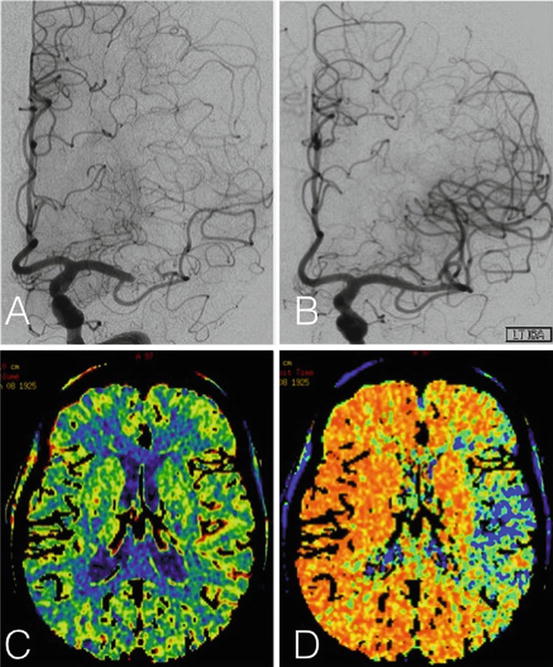

Fig. 5.3
Patient with near occlusion of M1 MCA who is symptomatic when her blood pressure is dropped. (a) Cerebral angiogram demonstrating the M1 MCA near occlusion before angioplasty and stenting, (b) cerebral angiogram after treatment with improved stenosis, (c) CBV (cerebral blood volume) increased in corresponding area, (d) MTT (mean transit time) decreased in corresponding area of symptoms and stenosis
Procedural Considerations
Patient Selection
Patients with recurrent symptoms in the setting of high-grade intracranial stenosis (>70 %) and maximal medical therapy (dual antiplatelet agents, high-dose statin treatment, glycemic control, normotension, regular aerobic exercise, and smoking cessation) may be candidates for endovascular therapy. Other considerations include the location of stenosis and mechanism of stroke. Stenosis in non-perforator-rich locations (intracranial internal carotid and vertebral arteries) is likely lower risk during endovascular treatment than perforator-rich locations (basilar and proximal middle cerebral arteries). Also, patients with hypoperfusion-related events would seem, based on physiology, most likely to benefit from improvement in luminal diameter. However, this later point has not been confirmed in the most rigorous clinical trials to date.
Pre-procedure
Once stenosis has been identified on noninvasive imaging, it is important to perform a stand-alone catheter angiogram for presurgical planning. A three-dimensional image is sometimes helpful to further characterize the lesion and optimal imaging angle to utilize during endovascular surgery. This diagnostic procedure also allows risk stratification by characterizing the proximal vessels and aortic arch as well as the degree of intracranial tortuosity that needs to be navigated in order to deliver the angioplasty balloon and/or stent to the desired location. The degree of angulation of the posterior and anterior genus of the cavernous carotid is a key determinant of success. Highly angulated carotid genus may prohibit the navigation of a stent distally. It is important to remember that endovascular treatment of ICAD is one of the highest-risk procedures an interventionalist will perform, and the strategy must be tailored to the patient and his/her individual anatomy.
As noted above, all patients being considered for endovascular treatment will have failed a course of dual antiplatelet agents (aspirin and clopidogrel) along with high-dose statin therapy. It is essential that these agents be continued pre- and post-procedure. Consideration should be given to pre-procedural platelet function testing to screen for aspirin or clopidogrel resistance. The utility of these tests in reducing procedurally related stroke is controversial, but given the high-risk nature of this treatment, all potential methods of risk reduction must be considered.
Anesthesia
Most neurointerventionalists currently perform intracranial angioplasty and stenting under general anesthesia. The arguments for this approach include the need for high-resolution imaging that is enhanced by decreased patient motion and mechanically induced apnea when needed. Elimination of the risk of sudden patient movement during a high-risk portion of the procedure is also achieved with general anesthesia. On the other hand, proponents of moderate sedation argue that the ability to monitor neurological function during the course of the procedure is invaluable. Additionally, the potential for medication-induced hypotension and subsequent hypoperfusion-related stroke may be less with moderate sedation. An arterial line should be in place both for procedural purposes and, just as importantly, for post-procedure blood pressure monitoring.
Sheaths
A 6F or larger sheath is needed to perform these procedures. A sheath length (35 cm or greater) that bypasses the ileac artery and distal abdominal aorta is recommended due to the high incidence of femoroiliac stenosis/tortuosity and abdominal aortic aneurysms in this population. When treating posterior circulation lesions, a radial access site may be advantageous. In this case a 6F 10 cm or shorter sheath is recommended. When intracranial stenting is planned, a 90 cm guiding sheath positioned in the distal common carotid artery or proximal internal carotid artery provides excellent support and allows the use of a “triaxial” system (guiding sheath, guiding catheter, and microcatheter) in the setting of difficult balloon/stent navigation.
Guiding Catheters
A 6F or larger guiding catheter is generally recommended. Standard guiding catheters can be safely positioned within the distal cervical carotid artery or at the V2/3 junction (e.g., Envoy XB; Codman Neurovascular; Raynham, MA). A 45-degree tip catheter may help guide the force vector during the procedure. Alternatively, a more flexible, atraumatic tip guide catheter may be navigated into the petrous/cavernous carotid (e.g., Neuron; Penumbra Inc; Alameda, CA). Some practitioners believe this more distal position outweighs the less supportive design of such catheters.
Intermediate Catheters
A relatively recent addition to the endovascular armamentarium is the intermediate or distal access catheter (DAC). These catheters can be routinely navigated into the cavernous or supraclinoid segment. This can greatly facilitate accessing middle cerebral artery stenotic lesions by bypassing the cavernous segment.
Microcatheters
A low-profile microcatheter and microwire are used to cross the stenotic segment. There are many equally effective microcatheters available for this purpose such as the SL 10 (Stryker; Kalamazoo, MI).
Microwires
Two microwires are often used. The first is a standard length 0.014″ wire that, along with a low-profile microcatheter, is used to cross the stenosis. Ideal wire characteristics include 1:1 torque and a soft tip (e.g., Synchro 14; Stryker; Kalamazoo, MI). A headhunter shape to the distal wire tip often facilitates crossing the lesion. The second wire is an exchange length 0.014″ wire. This wire should be supportive proximally and highly shapable distally (e.g., X-Celerator; Covidien; Irvine, CA). A J- or C-shaped wire tip will reduce the risk of micro-branch wire migration and perforation.
Balloons
Over-the-wire, semi-compliant balloons are generally preferred over monorail systems due to the greater trackability of the former through tortuous vessels. There is only one FDA-approved (HDE pathway) balloon for intracranial angioplasty in the setting of ICAD. The Gateway balloon (Stryker; Kalamazoo, MI) is an over-the-wire, low-profile, and highly navigable balloon. It comes in a variety of diameters and lengths. Coronary angioplasty balloons have also been used off label for this purpose (e.g., Maverick; Boston Scientific; Natick, MA). With the use of balloon-mounted stents, a pre-dilation with a smaller balloon may facilitate stent passage.
With self-expanding stents, pre-stenting balloon diameters are generally sized to 80 % or less of the normal luminal diameter. Balloon lengths are generally 5–10 mm greater than the lesion length. Extreme care must be taken to avoid overdistention of the vessel, as the fragile angio-architecture of the circle of Willis makes vessel rupture a real and catastrophic possibility.
Stents
The Wingspan Stent System is the only FDA-approved (HDE pathway) device for intracranial stenting in the setting of ICAD. It is used in conjunction with the Gateway balloon. These nitinol, slotted tube, self-expanding stents are housed within a delivery catheter. The delivery catheter is generally navigated over an exchange wire and across the lesion. The delivery catheter is then withdrawn, allowing the stent to flower open. The diameter of the stent is generally close to that of the normal luminal diameter. The length is generally 5–10 mm greater than the lesion length. Over-the-wire, balloon-mounted coronary stents, both bare metal and drug-eluting, have been used off label to treat ICAD. These devices are less navigable, but with a highly supportive proximal system, can generally cross the stenotic lesion. Stent sizing with these devices is 80 % or less of the normal luminal diameter.
Procedural Steps
Anesthesia is induced. A sheath is placed within the access site. Heparin is administered to achieve an activated clotting time of >250 seconds and rechecked hourly. The guide catheter is navigated into the parent vessel. A low-profile microcatheter and standard length microwire are navigated intracranially and, under high-resolution road map guidance, used to cross the stenotic lesion. These devices are positioned in a large branch, a sufficient distance distal to the stenosis to allow support and access during subsequent steps (e.g., in the M3 angular branch or P2/3 segment). The standard length microwire is removed and replaced by an exchange length microwire. The microcatheter is then removed over the exchange wire. This step takes extreme care in order to avoid sudden movement of the wire. Sudden forward movement is most dangerous as this may lead to wire perforation. A J- or C-shaped wire tip will reduce the tendency of the wire to enter small branch vessels in this circumstance. If the wire migrates proximally, trans-lesional access may be lost, or insufficient distal access may make balloon/stent navigation impossible. These exchanges generally require two operators. At this point a repeat high-resolution road map is helpful to illustrate any changes in vessel angulation caused by the microwire. Next an over-the-wire angioplasty balloon is navigated across the lesion and inflated (see discussion above for balloon sizing tips), slowly deflated, then removed from the arterial system.
If the Wingspan Stent System is being utilized, the delivery catheter is advanced over the exchange wire and across the lesion. Despite the hydrophilic coating and flexible design of this device, it is often a slow and laborious process to achieve the desired stent position across the stenotic lesion. The stent is deployed and the delivery catheter is removed (see above for stent sizing tips). An angiogram through the existing catheter is then performed. If insufficient luminal improvement is seen, a post-stent angioplasty can be performed, but is discouraged by the manufacturer.
If an over-the-wire, balloon-mounted coronary stent system is being utilized; a pre-stent angioplasty may not be needed, depending on the severity of the baseline lesion. Once the stent is navigated across the lesion, the balloon upon which it is mounted is inflated to nominal pressure (see above for stent sizing tips). A post-stent angioplasty can be performed if the desired luminal enlargement is not achieved. A final control angiogram will screen for thromboembolic complications or extravasation.
Post-procedural Considerations
It is important to carefully monitor and guard against spikes in blood pressure during the post-procedural period. High-risk points include awakening from anesthesia and extubation. Such spikes in blood pressure may precipitate hyperperfusion syndrome and ICH. Additionally, it is essential that dual antiplatelet therapy be maintained for at least 3 months. In the case of drug-eluting coronary stent use, 12-month to lifelong dual antiplatelet therapy is recommended.
Endovascular ICAD Studies
We have reviewed the literature on PTA, DES, BMS, and SES above. Despite having many options for treatment of ICAD with endovascular techniques, none has been established as primary treatment. Table 5.4 outlines some of the major literature on these approaches.
Table 5.4




Majora studies on endovascular treatment of intracranial atherosclerotic disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








