
Endovascular Treatment of Posterior Circulation Aneurysms
Objectives: Upon completion of this chapter, the reader should be able to explain the indications for and results with endovascular coiling of posterior circulation aneurysms, including ruptured, unruptured, and dissecting aneurysms.
Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians.
Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity.
The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp
* The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons.
In this chapter, we briefly review the natural history of both ruptured and unruptured posterior circulation aneurysms. We then present the latest data supporting endovascular treatment for both types of aneurysms.
 Intracranial Aneurysms: Epidemiological Data
Intracranial Aneurysms: Epidemiological Data
Crude annual incidence rates of aneurysmal subarachnoid hemorrhage (SAH) have been estimated at between 10 to 15 cases per 100,000, with gender, ethnic, and geographical variations.1 Approximately 15,000,000 Americans have an intracranial aneurysm; the exact number, however, is probably underestimated.2 Aneurysms of the posterior circulation range between 8 and 15% of all intracranial aneurysms, according to several authors.3–5 The majority of these aneurysms are located in the basilar apex, followed by the superior cerebellar arteries, the posterior inferior cerebellar arteries, the vertebrobasilar junction, the vertebral arteries, and the posterior cerebral arteries.
 Ruptured Aneurysms
Ruptured Aneurysms
Prognosis in Ruptured Posterior Circulation Aneurysms
In the United States, every year ~50% of the 28,000 patients with SAH secondary to ruptured intracranial aneurysms die or are severely disabled. It is well established that the outcome of all ruptured intracerebral aneurysms depends on several factors, such as (1) age, (2) accessibility to a care facility, (3) the patient’s clinical status (according to the Hunt and Hess grading system),6 (4) the amount of intracerebral hemorrhage (according to the Fisher’s grading scale), (5) aneurysmal location, and (6) the presence of vasospasm.
Access to a care facility is still a primary problem. Approximately 12% of patients die before reaching a hospital, 40% of patients expire within 30 days from the rupture, and ~30% of the survivors are left with major neurological deficits.7 In addition, between 5 and 15% of all patients presenting with the clinical symptoms of stroke are found to have SAH due to a ruptured intracranial aneurysm. Early treatment within 24 to 72 hours has been advocated for these patients because of the high risk of repeat rupture within the first 2 weeks of SAH.8
For ruptured posterior circulation aneurysms, the prognosis is even more dismal. According to Schievink et al, these patients can expect survival rates of 32% within the first 48 hours of hemorrhage, 11% within the first 30 days, and have mortality rates three times that of ruptured anterior circulation aneurysms9. The close proximity of the brainstem and the propensity for hemorrhage into and expansion of the fourth ventricle is one of the factors explaining their poor outcomes. In addition, the decreased level of consciousness that often follows the rupture of posterior circulation aneurysms prevents the patient from seeking care urgently.
Treatment of Ruptured Posterior Circulation Aneurysms
The surgical approach to these lesions with traditional microsurgical techniques is technically challenging and anatomically unfavorable. In particular, approach to posterior circulation aneurysms requires a considerable amount of brain retraction and frequent protracted arterial occlusion, thus resulting in significant morbidity and mortality even in the best surgical hands. Consequently, the poor surgical results for ruptured posterior circulation aneurysms led to early studies using endovascular embolization to provide at least some protection from early rebleeding.10
The endovascular approach to intracranial aneurysms was originally reserved for the treatment of large, non-surgical, giant aneurysms and used the method of endovascular “Hunterian” occlusion of the parent artery with detachable balloons.11,12 However, in 1991 Guido Guglielmi revolutionized the field of endovascular embolization of intracranial aneurysms with the development of detachable platinum coils.13,14 The first studies evaluating the endovascular treatment of aneurysms were largely descriptive and included patients with aneurysms that were considered too difficult to clip, had poor neurological status, had advancing age, or were otherwise predisposed to a poor overall prognosis.15 As a result, in the early experience with Guglielmi detachable coils (GDC) reported by Vinuela et al,16 57% of the 403 patients treated in eight centers in the United States had aneurysms of the posterior circulation, a percentage that far exceeds the expected range of 8 to 15%.3,4,15
In a study evaluating the treatment of acutely ruptured and unruptured aneurysms of the basilar tip, Raymond et al treated 23 patients with acutely ruptured aneurysms using GDC and prevented rebleeding in all patients (mean follow-up: 15.5 months)17. The mortality and poor outcome rates were 8.7% each, respectively, and were attributed to intracranial hypertension related to the initial SAH and vasospasm. One death and one minor permanent deficit, but with good outcome, were attributed directly to endovascular treatment. These results compared favorably to the largest series of surgically treated basilar tip aneurysms compiled by Peerless et al18 In this study of 1767 vertebrobasilar aneurysms, 113 were treated surgically within 1 week of SAH. The mortality (8%) and morbidity (9.7%) rates were related to the initial or recurrent SAH, vasospasm, or technical complications.
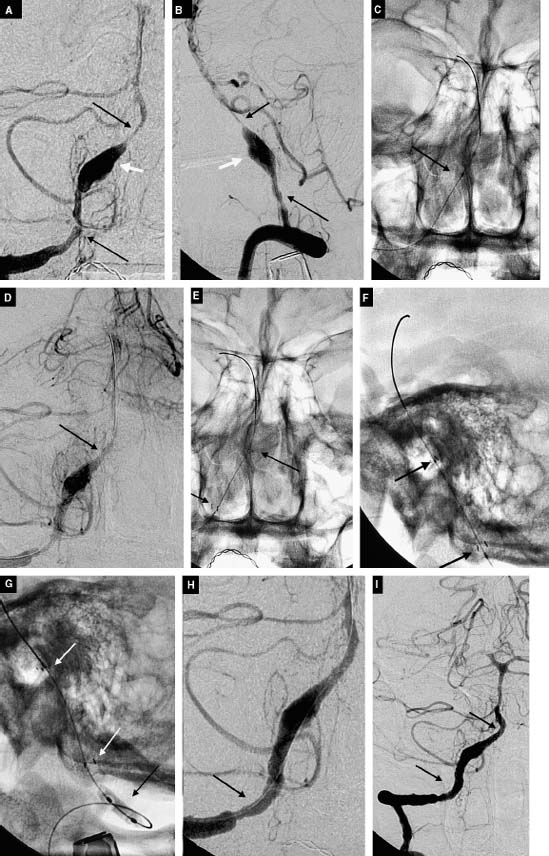
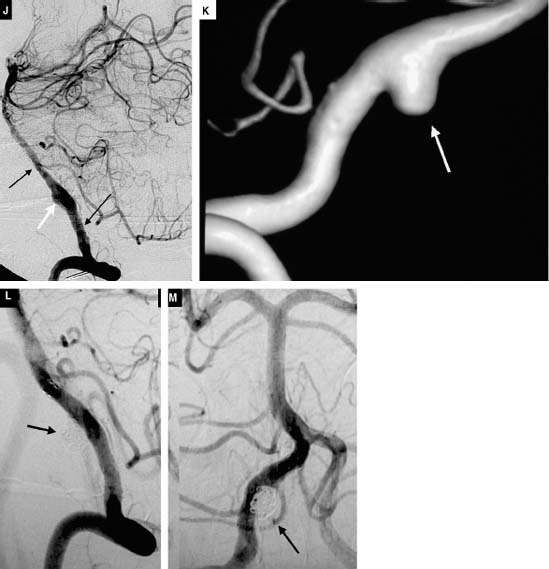
The rate of complete aneurysm obliteration by open neurosurgery for all aneurysms has been reported to be as high as 96%.19,20 The resulting surgical remnant with “failed” aneurysm surgery may result in a significant rate of repeat hemorrhage,20,21 estimated to be 0.8% per year22. Complete obliteration rates higher than 70% using endovascular coiling were once difficult to achieve even in a series consisting of small aneurysms (<10 mm) bearing small necks (<4mm)16. When aneurysms are large (10-25 mm) and giant (>25mm), obliteration rates drop to 57% and 50%, respectively. However, it has been reported repeatedly that long-lasting, complete obliteration of the aneurysm after coil embolization is likely related to packing density. With experience, operators have recorded a decreased frequency of aneurysm neck remnants and increased the degree of coil packing. These factors have shown to play a major role in decreasing the likelihood of recanalization. Another critical factor in determining the frequency of recanalization is a neck size greater than 4 mm16. The literature suggests the rate of endovascular remnant rebleeding to be 0%, 4%, and 33% for patients with treated small, large, and giant aneurysms, respectively (average 3.5-year follow-up) .23 However, the advent of bioactive coils as well as of balloon- and stent-assisted coiling techniques continues to reduce these remnant rates (Fig. 11-1A-C, Fig. 11-2A-F). In addition, the high rates of aneurysm recurrence with endovascular therapy ought to be considered in light of (1) lower morbidity and mortality associated with endovascular therapy, (2) a rapidly advancing coil technology that is allowing safer and higher packing density, and (3) the ability to safely retreat these remnants at follow-up evaluation (Fig. 11-3A-M).
Cerebral vasospasm is the most common cause of morbidity and mortality in patients admitted to the hospital after developing SAH. The early surgical removal of subarachnoid clots and irrigation of the basal cisterns have been reported to reduce the incidence of vasospasm. In contrast to surgery, endovascular treatment of aneurysms does not allow removal of subarachnoid clots; therefore, it was considered less beneficial. However, Murajama et al measured the incidence of symptomatic vasospasm after early endovascular treatment of acutely ruptured aneurysms with GDCs24. Sixty-nine patients classified as Hunt and Hess Grades 1 to 3 underwent occlusion of intracranial aneurysms via GDCs within 72 hours of rupture. The amount of blood on the initial computerized tomography (CT) scan was classified by means of the Fisher’s scale. Symptomatic vasospasm was defined as the onset of neurological deterioration verified with angiographic or transcranial Doppler studies. At 6-month clinical follow-up examination, 12 of these 16 patients experienced a good recovery, 2 were moderately disabled, and 2 had died of vasospasm. The authors concluded that the 23% incidence of symptomatic vasospasm in this series compares favorably to that found in conventional surgical series of patients with acute aneurysmal SAH.
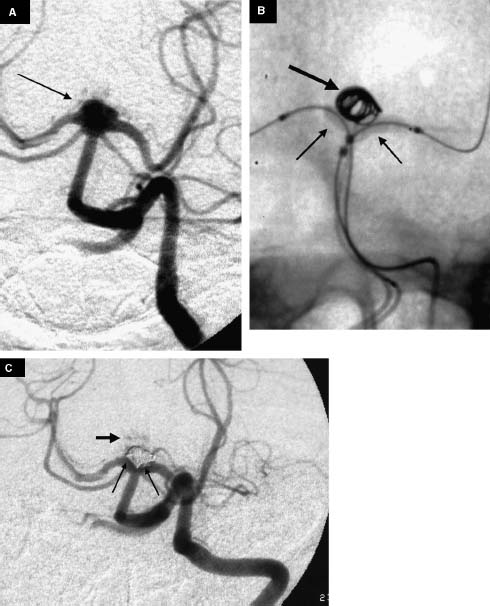
FIGURE 11-1 A 60-year-old woman with an unruptured basilartip aneurysm. (A) Left vertebral artery (VA) injection shows a broad-base basilar tip aneurysm (arrow). (B) Placement of two non-detachable balloons from both VAs (small arrows) and coiling (large arrow) of the aneurysm via a microcatheter placed into the right VA. (C) Left VA injection shows complete aneurysmal obliteration with preservation of the perforating arteries (large arrow). In addition, the angiogram shows balloon-induced coil mass remodeling. There is a new coil interface with the aneurysmal base and the left P1 segment of the posterior cerebral artery (long arrows).
Comparison of Open Surgery with Endovascular Treatment: Evidence from Randomized Trials
The first randomized clinical trial to compare open surgery with the endovascular treatment of acutely ruptured aneurysms was published in 1999.25 The prospective randomized study consisted of 109 patients with SAH, an identified aneurysm, and potential for treatment using either modality. After treatment, the patients were followed clinically (3 months post SAH), angiographically (3, 12, and 36 months), and with neuropsychological testing (3 and 12 months). The technical mortality rate was 4% in the surgical group and 2% in the endovascular group. Significantly better initial angiographic results were achieved after endovascular treatment of patients with posterior circulation aneurysms (N= 11; p= 0.045).
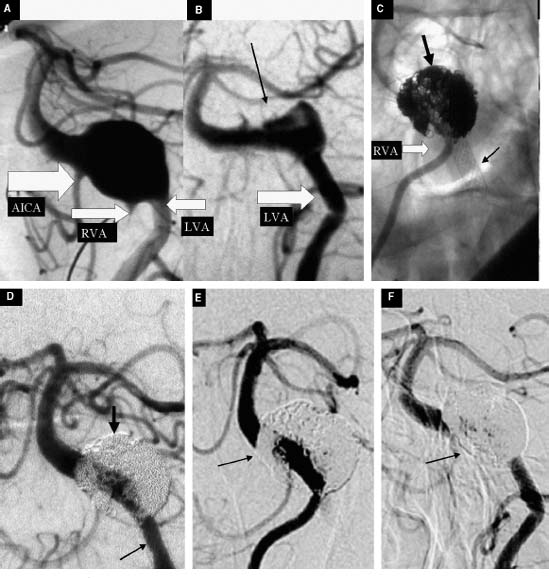
FIGURE 11-2 A 78-year-old man with a giant fusiform aneurysm of the vertebrobasilar junction, giving rise to headaches—a VI cranial nerve palsy. (A) Oblique view of the angiogram obtained with a left vertebral artery (VA) injection. The anterior inferior cerebellar artery (AICA) and the right and left VAs are indicated by the white arrows. The patient was initially treated with three low-porous stents (Magic Wallstent, Boston Scientific BSC, Natick, Massachusetts) deployed under the fusiform aneurysm via the left VA (B) Four-week follow-up angiogram obtained with a left VA injection shows partial thrombosis of the lesion (black arrow). The angiogram also shows straightening of the distal segment of the left VA and proximal segment of the basilar artery secondary to placement of the three stents. (C) Unsubtracted oblique view of a right VA injection shows coil embolization (large arrow) through the stent struts and the stents (small arrows). (D) Final oblique view of the left VA injection after coil embolization. (E, F) Sixteen-month follow-up angiogram with similar oblique view obtained with right (E) and left (F) VA injection that shows arterial remodeling and aneurysm obliteration (small arrows) with preservation of the parent vessel.
Recently, the International Subarachnoid Aneurysm Trial (ISAT) provided level I evidence on the benefits of endovascular versus surgical treatment of ruptured intracranial aneurysms.26 The study was a randomized, controlled trial of surgical clipping versus endovascular coiling for ruptured intracranial aneurysms. The multicenter study enrolled 2143 patients with aneurysms that were deemed suitable for either treatment modality. Random assignment included 1070 patients for clipping and 1073 patients for coiling. More than 50% of the aneurysms were less than 6 mm, and over 90% were less than 10 mm in diameter. Interestingly, the majority of aneurysms enrolled in the study involved the anterior cerebral artery (50.5%), followed by the internal carotid artery (including posterior communicating artery aneurysms). Only a small number of aneurysms were located in the posterior circulation (2.7%). The fivefold reduction in the number of posterior circulation aneurysms (8 to 15% expected) reflects a selection bias. This is because the ISAT adjudicating committee considered it unethical to randomize posterior circulation aneurysms. Both neuroradiologists and neurosurgeons agreed that the majority of these aneurysms should be treated by coil embolization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


