ENT Bleeding and Tumor Embolization
Key Points
Airway protection is one of the biggest oversights or risk factors affecting patient safety and outcome during head and neck embolizations.
Hazardous anastomotic connections in the region of the orbit and at the skull base are almost universally present to some degree and need to be considered carefully during craniofacial embolizations.
Head and neck embolizations for acute bleeding, preoperative tumor devascularization, or preoperative test occlusion share a common anatomic background and similar considerations with reference to technology and risk, and, therefore, to avoid repetition will be discussed together here. Fatal or debilitating complications from these procedures are rare, fortunately. Most patients are remarkably resilient and can pull through anything reasonable that the medical profession is apt to throw at them. It is with an uncommon or rare event that one’s experience or diligence will make a difference to patient outcome. Adverse endovascular outcomes in the head and neck involve blindness, disfiguring skin necrosis, or intracranial ischemic strokes, so one has to conduct these and all neurointerventional procedures methodically and carefully with a view to preventing a rare or uncommon set of circumstances whereby such outcomes arise. There are two principal considerations for patient safety during these procedures.
The first consideration is airway protection. A patient who is propped up in bed talking to you before the procedure may have much more difficulty maintaining an airway when sedated and supine on the angiographic table. Furthermore, if bleeding resumes during the procedure into the oropharnygeal cavity this may escape the initial attention of staff in the room and the patient may easily aspirate a large clot. Clotted blood is much more difficult for patients to clear from the airway than phlegm or other secretion, and respiratory distress can quickly establish itself. Moreover, patients who have had laryngeal surgery may not be able to communicate their distress to the team, particularly when they are hidden from view under the drapes and behind the lead shields. Therefore, a low threshold for using general anesthesia is recommended. It is a tedium waiting for the anesthesia team to arrive and to get all their lines set up, and the temptation is there to say that one can get along perfectly fine without them. That is true in 98% of cases, but it is the other 2% that counts.
Secondly, many disastrous or unnecessary complications of head and neck embolization procedures relate to variant or misperceived vascular anatomy. One must be very suspicious at all times that variant connections with the ophthalmic artery or intracranial vessels might be present, even if they do not show themselves on the initial angiogram. Dynamics of flow can change with relief of catheter-induced vasospasm or due to the effects of progressive embolization, meaning that injections that appeared safe early in the procedure become much more hazardous later. The commonest dangerous anastomotic junctures (1) where the external carotid and intracranial circulation interplay are:
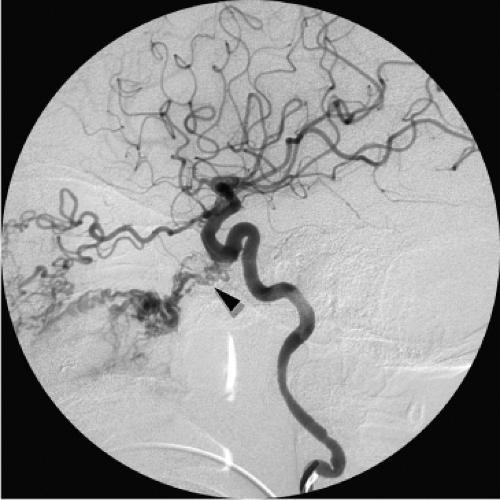
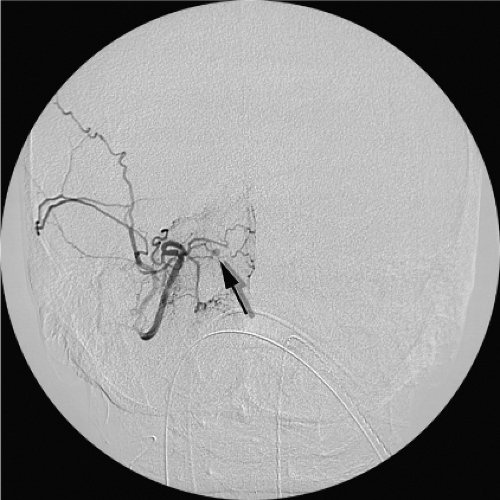
The common carotid artery bifurcation. During all embolizations in proximal branches of the external carotid artery the risk of inadvertent reflux of an errant coil or liquid agent into or even close to the external carotid artery trunk must be kept in mind. The biphasic pattern of flow in the external carotid trunk can pull a liquid device or loose coil into the stream of common carotid flow and thence to the head (Fig. 25-1).
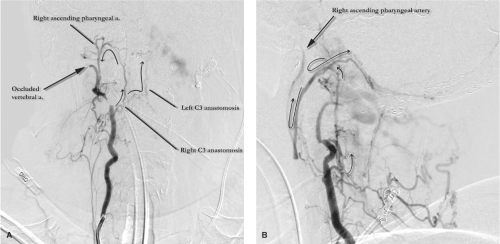
The ascending pharyngeal artery. The anastomotic connections of the ascending pharyngeal artery are many, and include the C3 anastomosis to the ipsilateral vertebral artery and laceral branch to the inferolateral trunk of the internal carotid artery, amongst others (Fig. 25-2).
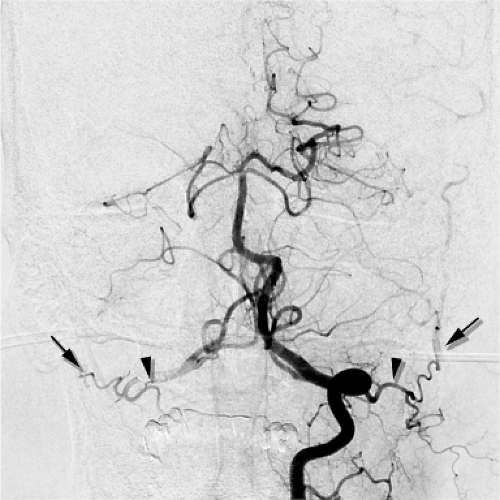
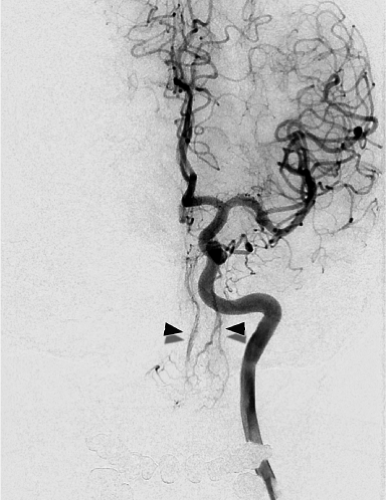
The C1 and C2 anastomotic connections between the vertebral artery and the occipital artery. These are consistently present in virtually all patients and are sometimes characterized by an immediacy of flow that can be alarming. Embolizations in the occipital artery should be conducted with an understanding that the ipsilateral vertebral artery is only millimeters away and readily accessible to the contents of one’s syringe (Fig. 25-3).
The middle meningeal artery and ophthalmic artery. The variant connections whereby the ophthalmic supply is partially or completely derived from variant branches of the middle meningeal artery have been covered in
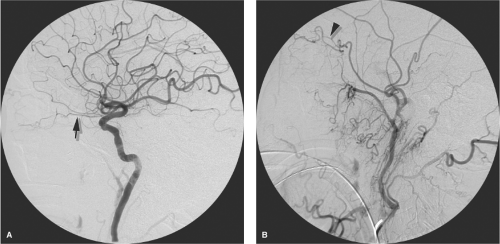
previous chapters. Orbital pathology, such as vascular malformations, will tend to accentuate the likelihood of such anastomoses opening up to a physiologically significant degree. A good habit to cultivate is that one’s first glance at every external carotid arteriogram should be for the presence of any component of the ophthalmic artery (Figs. 25-4 and 25-5).
Distal external carotid artery and internal carotid artery. Anastomotic connections between the terminal branches of the external carotid artery and the intracranial circulation can flow readily via such routes as the foramen rotundum and the Vidian canal. Even when they are not seen angiographically, one has to bear them in mind when
using hazardous agents such as Onyx, nBCA, or small particles (Fig. 25-6).
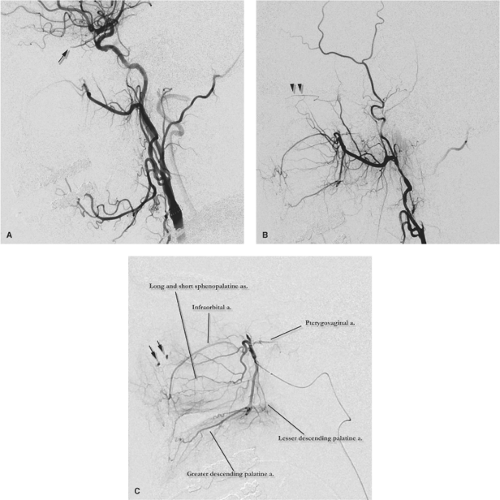
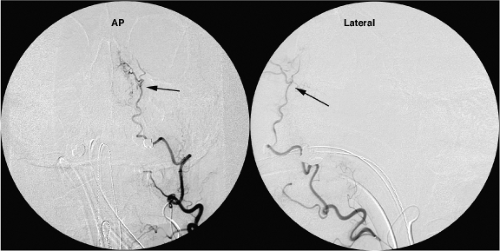
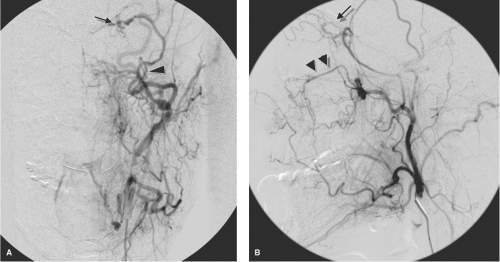 Figure 25-4. (A–B) Ophthalmic artery (arrow) supplied by middle meningeal artery (arrowhead). This young patient demonstrated bilateral anomalous supply to the ophthalmic artery from the middle meningeal artery, unrecognized by the physician performing an epistaxis embolization, as a result of which she lost vision in one eye (see Figure 5-1). The AP (A) and lateral views (B) of the left external carotid artery show the ophthalmic artery territory (arrows) perfused from the middle meningeal artery (arrowhead in A). The infraorbital artery (double arrowheads in B) serve as a reference for the orbital anatomy. |
Epistaxis and Sinonasal Bleeding
Severe epistaxis of a degree warranting embolization is usually of the idiopathic posterior type, although this procedure will also be requested for epistaxis following trauma and epistaxis associated with tumor erosion, postsurgical complications, bleeding diathesis, and conditions such as hereditary hemorrhagic telangiectasia (HHT).
Posterior idiopathic epistaxis is a fulminant arterial type of bleeding seen in hypertensive adult patients and is thought to be related to a denudation of the nasal mucosa over the sphenopalatine arteries in the posterior nasal cavity. It is quite distinct from the minor venous oozing seen in children or young adults where the bleeding is coming from Little’s area (Kiesselbach plexus) along the anterior nasal septum. The volume of bleeding from posterior epistaxis can be life-threatening, particularly when there are comorbidities. Primary treatment for posterior epistaxis consists of first-aid measures such as application of external pressure, endoscopic cauterization of the bleeding point, and packing of the nasal passage with gauze maintained under pressure with a clamped Foley balloon catheter (2). This is a most uncomfortable sensation for the patient, and most will willingly consent to endovascular embolization of the epistaxis if it involves the promise of expeditious removal of the balloon.
Embolization for control of epistaxis was described in 1974 by Sokoloff et al. (3) and has a technical success rate for controlling the bleeding of over 85% (4,5,6,7,8). Minor complications from the procedure occur in 8% to 21% of patients. While major complications such as neurologic injury, septal perforation, blindness, CVA, or skin necrosis have been reported, the incidence is low—0% to 1.4% (9).
As mentioned previously, the incessant dripping of blood into the nasopharynx through the packing material
represents a serious risk of aspiration and airway compromise for a patient who is about to undergo a procedure involving several hours supine under sedation. General anesthesia for airway protection is a prudent investment of time and resources for such patients.
represents a serious risk of aspiration and airway compromise for a patient who is about to undergo a procedure involving several hours supine under sedation. General anesthesia for airway protection is a prudent investment of time and resources for such patients.
As the prototypical neurointerventional “easy” case, epistaxis embolization exemplifies many of the procedures of this discipline in that it can be performed with a generous degree of latitude for safety and freedom from complications in most patients. However, this impression is deceptive. The potential for injury to the patient through use of inappropriate devices and materials, incorrect embolization technique, or lack of awareness of dangerous collateral branches to the internal carotid artery or ophthalmic artery is greater than the neophyte or pinch hitter neurointerventionalist-for-a-day supposes, and the learning curve, even for epistaxis embolization, is longer in retrospect than is apparent to the beginner with a half-dozen cases notched on his fuselage. Nevertheless, epistaxis embolization is a good starting point for a discussion of elementary techniques and use of polyvinyl alcohol (PVA) and Gelfoam, and how things can always go wrong even in the most foolproof situation.
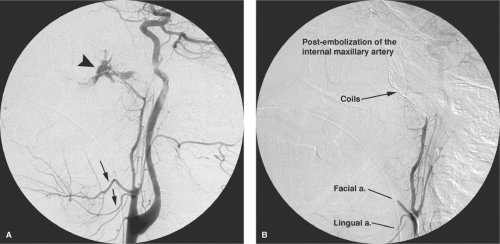
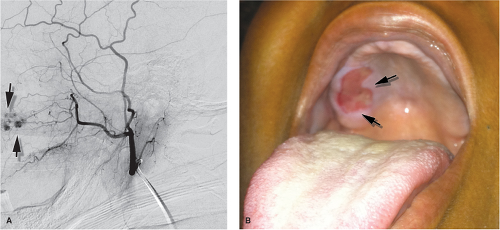
The angiographic evaluation starts on the side ipsilateral to the bleeding and requires some initial review of the proximal intracranial internal carotid artery to ascertain that the bleeding is not from some pathology of the internal carotid artery (a very rare but important consideration) (10) and to evaluate the prominence of the ethmoidal branches of the ophthalmic artery. Should the ethmoidal branches be dominant in the supply of the nasal septum, resumption of epistaxis after an otherwise successfully accomplished embolization may imply that surgical clipping of the ethmoidal branches may be necessary to control the bleeding (Fig. 25-7). Next, angiography of the external carotid artery is performed to map the anatomy of the internal maxillary artery, which is then navigated with a microwire and microcatheter to a point ideally just distal to the origin of the infraorbital artery. In reality, the infraorbital branch is often included in the embolization field either directly, for want of being able to catheterize the internal maxillary artery more distally, or through reflux of particles (Figs. 25-8–25-10). An angiographic run via the microcatheter in the internal maxillary artery runs the risk of redundancy at this point but it is, nevertheless, prudent to exclude the possibility of hitherto invisible critical collaterals. If all is well, the internal maxillary artery is then embolized carefully to the point of near stagnation of flow with PVA particles (250 to 1,000 μm) suspended in contrast. The vessel can be plugged finally with a pledget or two of Gelfoam. Coils should not be used in an epistaxis embolization because they are of limited efficacy in this instance and, more importantly, deny the possibility of a repeat procedure on that side if the patient should bleed again at a later date.
The facial artery—or very rarely the transverse facial artery—ipsilateral to the side of nasal bleeding may have the capacity to collateralize flow to the nasal mucosa of the lateral nasal wall via its angular branch (Fig. 25-11). Therefore, it is common to perform a very light (2 to 3 mL) particle embolization of the ipsilateral facial artery to suppress this tendency. However, this has to be done lightly to avoid the possibility of nasal skin or jugal necrosis in a field that
has already been compromised through embolization of the infraorbital artery via the internal maxillary artery.
has already been compromised through embolization of the infraorbital artery via the internal maxillary artery.
Finally, the contralateral internal carotid artery and external carotid artery are studied as above. The internal maxillary artery is catheterized and embolized with PVA and Gelfoam (Figs. 25-12–25-17) as on the previous side. The facial artery is not touched. For other causes of posttraumatic or postsurgical epistaxis, the embolization technique may have to be varied accordingly, following principles discussed in Box 25-1) that are more appropriate to emergency head and neck bleeding. In such cases, use of coils, for instance, might be entirely favorable because the lacerated vessel may be more proximal than those involved in posterior epistaxis (Figs. 25-18 and 25-19).
Hypervascular Tumors of the Head and Neck
Embolization of head and neck tumors is frequently undertaken because of the surgeon’s concern about inordinate or uncontrollable blood loss during proposed surgery. Most of these cases are tumors idiosyncratic to this anatomic region, juvenile angiofibromas and paraganglioma, but other occasions present themselves such as metastatic thyroidal carcinoma or renal cell carcinoma.
 Figure 25-14. Rolling a pledget. Tiny torpedoes are rolled as illustrated (but only with a dry clean glove). |
Box 25-1 Polyvinyl Alcohol and Gelfoam
PVA particles are the staple of tumor and mass preoperative embolization in the head and neck. They are used only rarely now for transarterial embolization of arteriovenous malformations (AVMs) of the brain and dura because their effect is temporary.
Various commercial preparations of PVA are available, which are supplied in sizes between 45 and 1,000 μm. PVA particles of 50 to 150 μm have a more effective histopathologic outcome for inducing hemostasis and tumor necrosis than larger particles (11). However, this size range probably carries a greater risk of cranial neuropathy if inadvertent embolization of vasa nervorum occurs. Particles of this range could also cause skin and mucosal necrosis. Depending on operator preference, particle sizes of 250 to 750 μm are most commonly used in head and neck embolization because they constitute an adequate compromise between the need to penetrate the vascular bed of a preoperative tumor, on the one hand, and the need to avoid ischemic necrosis of target tissue and possible delay of healing in the surgical bed, on the other hand.
Particles are prepared as a dilute suspension in radiographic contrast and injected as punctuated miniboluses that are monitored on a fluoroscopic roadmap while being carried to their destination by antegrade arterial flow (Fig. 25-12). Careful monitoring of the pattern of flow is maintained throughout the embolization to avoid reflux of particles and to identify the development of vasospasm or opening of potentially dangerous anastomoses.
In situations in which small anastomoses are suspected or arteriovenous shunting is evident on the angiogram, larger particles may be preferred. The chances of obstructing a microcatheter with clumps of particles are greater with bigger particles. Microcatheters of the 0.010″ series are usually limited to PVA particles up to 250 μm.
Biologic Effects of PVA
PVA particles impair blood flow by adherence to the vessel wall in clumps, with preservation of some luminal flow in many vessels (12,13). They also provoke variable degrees of acute inflammatory reaction. Foreign body giant-cell reaction, mural angionecrosis, and necrotizing vasculitis can be seen in the acute phase (days) after embolization of AVMs and other lesions. However, the behavior of PVA is still relatively inert compared with other embolic agents, particularly compared with acrylate materials (14).
After PVA embolization of an AVM, there is usually preservation of some flow in large- and medium-sized vessels, and a tendency of embolized territories to recanalize slowly, over a period of weeks to months (11,15). This has suggested to some authors that PVA embolization of brain AVMs provides only a temporary improvement in the angiographic appearance. The size of an AVM nidus may be underestimated after PVA embolization. Radiotherapy planning based on this angiographic appearance runs the risk of recurrence of the AVM in those regions not included in the radiation port (9). The preservation of some minimal degree of flow in a tissue bed embolized with PVA is an important safety feature of this embolization agent. Embospheres, an alternative particle embolic material marketed for fibroid embolization, has been used in the head and neck for epistaxis and other embolizations but has an anecdotally higher risk of tissue necrosis due to the lubricious smooth texture of the particles and its tendency to occlude completely a target vessel.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree