and Héctor H. García3, 4
(1)
School of Medicine, Universidad Espíritu Santo, Santo, Ecuador
(2)
Department of Neurological Sciences, Hospital-Clinica Kennedy, Guayaquil, Ecuador
(3)
Cysticercosis Unit, Instituto Nacional de Ciencias Neurológicas, Lima, Peru
(4)
School of Sciences, Universidad Peruana Cayetano Heredia, Lima, Peru
Abstract
Taenia solium establishes its life cycle in regions where domestic pig raising coexists with poor sanitary conditions. Unfortunately, most poor areas of the world, with exception of Muslim populations, fulfill these simple requirements (Gilman et al. 1999). In poor regions, domestic pig raising is a usual component of survival economies, driven as a major incentive by the fact that pigs can be just left to roam by themselves and thus no money or effort need to be spent in food or forage, resulting in a very cheap production. Furthermore, there are very active commercialization systems for pigs and pork in most towns (Cysticercosis Working Group in Peru 1993).
Taenia solium establishes its life cycle in regions where domestic pig raising coexists with poor sanitary conditions. Unfortunately, most poor areas of the world, with exception of Muslim populations, fulfill these simple requirements (Gilman et al. 1999). In poor regions, domestic pig raising is a usual component of survival economies, driven as a major incentive by the fact that pigs can be just left to roam by themselves and thus no money or effort need to be spent in food or forage, resulting in a very cheap production. Furthermore, there are very active commercialization systems for pigs and pork in most towns (Cysticercosis Working Group in Peru 1993).
The World Health Organization has drawn an official map showing areas where Taenia solium cysticercosis is endemic (Fig. 4.1). These include most Latin American countries with the exception of Argentina and Uruguay, some islands of the Caribbean basin including Haiti and Dominican Republic, the sub-Saharan Africa (Benin, Burkina Faso, Burundi, Cameroon, Chad, Congo, Ghana, Kenya, Madagascar, Mozambique, Nigeria, Rhodesia, Rwanda, South Africa, Tanzania, Togo, Uganda, Zambia, Zimbabwe), the Indian subcontinent, and vast regions of Southeast Asia (Burma, China Laos, Indonesia, Nepal, Philippines, Thailand, and Vietnam) (O’Neal et al. 2012; Preux and Druet-Cabanac 2005; Schenone 1982; Winkler et al. 2010).
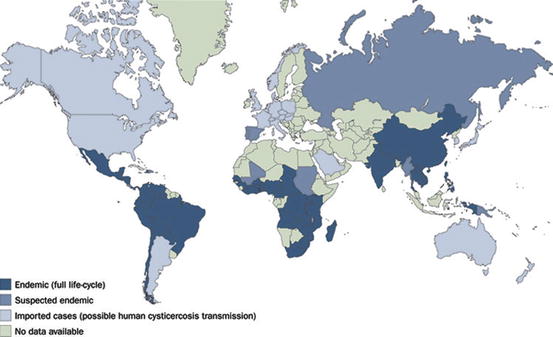

Fig. 4.1
World map showing areas where cysticercosis is endemic
Even in disease-endemic countries, marked differences in transmission and prevalence of Taeniasis and cysticercosis exist depending on the particular population group studied. Data from rural areas is not necessarily comparable to data from urban metropolis, and of course, data from hospital-based registries should not be compared with data from the general population (García and Del Brutto 2005). In a given population, there will be a series of diverse subgroups of infected persons in terms of symptomatic cases or serological or imaging evidence of cysticercosis (Fig. 4.2). On this basis, it is possible to differentiate among infected and noninfected individuals and on whether infections are symptomatic or not (Table 4.1). There are some limitations in this categorization, mainly arising from the fact that imaging is mostly restricted to diagnose neurocysticercosis.
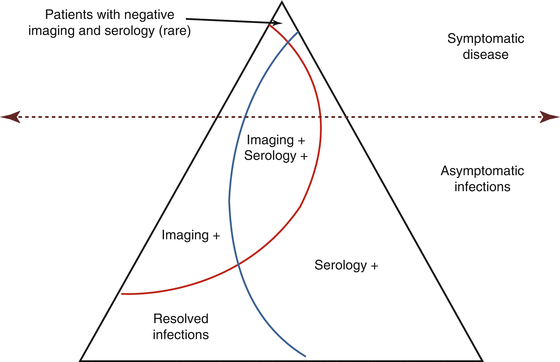

Fig. 4.2
Diagram showing gross prevalence of symptomatic and asymptomatic cysticercosis infections according to positive or negative results on neuroimaging studies and serology
Table 4.1
Subgroups of infected persons in terms of symptomatic cases or serological or neuroimaging evidence of cysticercosis
Not infected | Infected | ||
|---|---|---|---|
Asymptomatic | Symptomatic | ||
Negative for antibody, antigen, and imaging | + | ||
Exposed: antibody positive, antigen negative, CT negative | + | + | |
Infected, no viable lesions: calcifications on neuroimaging, antigen negative (independent of antibody results) | + | + | |
Infected, degenerating lesions: transitional, enhancing cysts on neuroimaging (often a single lesion) | ± | + | |
Infected, viable lesions: cysts on neuroimaging or positive antigen, often antibody positive | + | + | |
4.1 Data on Asymptomatic Individuals/General Population
4.1.1 Rates of Infection
As noted, assessing the prevalence of cysticercosis may be difficult due to the high percentage of asymptomatic infections. The most common ways to evaluate prevalence in rural endemic populations are by serology (anticysticercal antibodies or cysticercal antigens detection) or, rarely, by neuroimaging.
4.1.1.1 Anticysticercal Antibody Prevalence
Many surveys exist in the literature and of course the estimates vary according to the sensitivity and specificity of the test used. Before the introduction of the enzyme-linked immunoelectrotransfer blot (EITB), the low sensitivity and specificity of the existing tests made it difficult to assess the extent of infection in endemic regions. Woodhouse et al. (1982) reported an overall 1 % of seropositivity in 20,000 samples from Mexico; with some communities reaching 6 %, the sensitivity and specificity of the test used in that survey—immunoelectrophoresis—were estimated to be around 50 %. Thereafter, some population-based studies used the enzyme-linked immunosorbent assay (ELISA) with varied results. Coker-Vann et al. (1981) reported seroprevalence rates ranging from 0.2 to 8.4 % in asymptomatic individuals living in different regions of Indonesia, which contrasted with the 16 % seroprevalence rate of asymptomatic individuals living around the Wissel Lakes area, where cysticercosis was highly endemic. In the latter, patients with diagnosis of neurocysticercosis had a seroprevalence of 61 %. In another study performed in rural Madagascar, 18 % of healthy individuals versus 36 % of neurocysticercosis patients had a positive ELISA in serum (Michel et al. 1993). Posterior studies, showing the disappointing reliability of the serum ELISA, make these surveys of historical importance only.
Once the highly specific EITB using purified lentil lectin glycoprotein antigens was available, multiple surveys have demonstrated that from 8 to 25 % of “healthy” persons living in endemic villages have antibodies to Taenia solium (García et al. 1991; Sarti et al. 1992). Asymptomatic seropositive individuals mostly present weak reactions to only one to three antibody bands (GP50, GP42-39, and GP24) (Schantz et al. 1994). In field conditions, antibody seroprevalence rates may overestimate the actual prevalence of infection because persons with anticysticercal antibodies resulting from exposure or from past infections are also detected. Nevertheless, antibody prevalence can give a general assessment of the levels of transmission and help to orientate control strategies. At the individual level, detection of specific anticysticercal antibodies in an asymptomatic person has limited clinical use.
4.1.1.2 Cysticercal Antigens Prevalence
More recently, some population-based data using circulating cysticercal antigen detection has become available. The prevalence of circulating antigen ranges from 1 to 22 % (Dorny et al. 2012; Mwanjali et al. 2013; Mwape et al. 2013; Nguekam et al. 2003; Praet et al. 2010). Antibody prevalence is usually much higher than antigen prevalence in these studies. There is no information about the predictive value of a positive antigen test in asymptomatic individuals or in the general population, neither is it known whether the use of a higher cutoff would improve its predictive value for neurocysticercosis. As for community-based antibody surveys, the application of antigen detection in an asymptomatic individual would likely be of no practical use, although its use for early detection of extraparenchymal infections has been suggested (García et al. 2012).
4.1.1.3 Prevalence Using Neuroimaging
The proportion of asymptomatic individuals with neurocysticercosis detected by computed tomography (CT) in endemic populations ranges from 10 to 25 % (Del Brutto et al. 2005; Fleury et al. 2003; Garcia-Noval et al. 1996, 2001; Medina et al. 2005; Montano et al. 2005; Sánchez et al. 1999) (Table 4.2). A single study in a high-risk population using magnetic resonance imaging (MRI) reported 5 % of people harboring small viable cysts, usually a single one (Prasad et al. 2011)
Table 4.2
Epidemiologic surveys evaluating the proportion of asymptomatic infections/symptomatic neurocysticercosis (documented by neuroimaging) among persons with epilepsy in endemic areas
Proportion with NCC on neuroimaging | Ratio of NCC in individuals with epilepsy/general population | ||
|---|---|---|---|
Location (author) | Asymptomatic individuals | People with seizures or with epilepsy | |
Guatemala (Garcia-Noval et al. 1996) | 12/51 (24 %) | 36/76 (47 %) | 2.01 |
Ecuador (Cruz et al. 1999) | 17/118 (14 %) | 14/26 (54 %) | 3.74 |
Honduras (Sánchez et al. 1999) | 29/144 (20 %) | 2/4 (50 %) | 2.50 |
Mexico (Fleury et al. 2003) | 14/153 (9 %) | 0/1 | … |
Peru (Montano et al. 2005) | Seronegative: 8/58 (14 %) | 15/39 (38 %) | 2.00a |
Seropositive: 18/53 (34 %) | |||
Ecuador (Del Brutto et al. 2005) | 1/19 (5 %) | 5/19 (26 %) | 5.00 |
Tanzania (Winkler et al. 2009) | 10/198 (5 %) | 38/212 (18 %) | 3.54 |
A closer view to the results showed in Table 4.2 provides useful insights. Field studies using CT consistently demonstrate a significant proportion of villagers harboring one or a few brain calcifications, more rarely multiple calcifications or even more rarely viable cysts. In these same field conditions, most asymptomatic, antibody-positive people show weak reactions. The positive predictive value of a positive antibody test in asymptomatic individuals is around 35 %, and the imaging correlate in such cases is usually one or a few parenchymal brain calcifications. The relative frequency of viable cysts in general population is low. Not all cases of neurocysticercosis are detected by an antibody assay, since a proportion of seronegative villagers will also show calcifications on brain CT (Montano et al. 2005). Overall, approximately 50 % of asymptomatic individuals with parenchymal brain calcifications are EITB negative. A proportion of villagers will have a positive antigen test, apparently fewer than those who are antibody positive. The positive predictive value of a positive antigen test in an asymptomatic individual is not well known and has been reported to be from 14 to 53 % (Mwanjali et al. 2013; Nguekam et al. 2003). The neuroimaging correlation for a positive result in antigen detection is usually that of multiple viable parenchymal brain cysts. It is unclear whether the remaining antigen-positive, neuroimaging-negative individuals may have viable brain cysts not detected by imaging or cysts elsewhere in their body.
4.1.1.4 Factors Associated to Infection in Field Settings
Diverse epidemiological studies and clinical series have demonstrated associations between positive tests and a history of intestinal taeniasis, pig raising, having seen cysticercosis in their pigs, and other more generic factors such as living in a rural area and poor sanitation. A history of intestinal taeniasis in the person or in the household is a very strong risk factor. The accuracy of questioning for an antecedent of taeniasis could be improved by showing villagers a proglottid, since patients who had expelled nematodes may assume that the question refers to this antecedent and answer affirmatively. Pig raising is a poorly specific indicator since in many villages more than half of the villagers raise pigs (Diaz et al. 1992; Garcia-Noval et al. 1996; Sarti et al. 1994). Having seen cysticercosis cysts in pigs (or in their own pigs) could provide a more specific marker, but we have not been able to find comparative assessments of the predictive value of these questions. There seems not to be particularly clear differences in the rates of infection be gender. In contrast, the prevalence of antibody-positive individuals in the general population begins to rise between 6 and 10 years of age and increase progressively with higher rates between 20 and 50 years of age (Diaz et al. 1992; Schantz et al. 1994).
4.1.1.5 Geographic Pattern/Clustering
Clustering of human and porcine cases around a tapeworm carrier was first described by Sarti-Gutierrez et al. (1988). Using GPS, Lescano et al. (2007) confirmed this and described that a stronger concentrically gradient is found for porcine seroincidence as an indicator of more recent transmission. Seizure cases, however, are not clustered around the tapeworm carrier, likely reflecting a clinically silent period between infection and seizures longer than the average life span of the adult tapeworm (Lescano et al. 2009). Familiar aggregation of cases is likely due to common source exposure, although some sort of genetic predisposition to infection or disease cannot be ruled out. The polymorphism of toll-like receptor 4 has been shown to be associated to symptomatic disease in human neurocysticercosis (Verma et al. 2010), and differences in some HLA determinants between patients and controls were demonstrated long ago in Mexico (Del Brutto et al. 1991).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







