Chapter 10 Epilepsy
Electroencephalogram (EEG)
Normal and Abnormal
The routine EEG records cerebral electrical activity detected by “surface” or “scalp” electrodes (Fig. 10-1). Four frequency bands of cerebral activity, represented by Greek letters, emanate from the brain (Table 10-1).
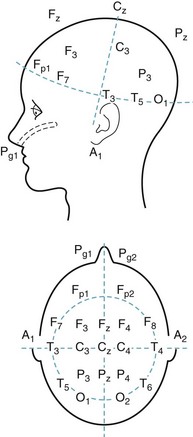
FIGURE 10-1 In the standard array of scalp electrodes, most are named for the underlying cerebral region (e.g., frontal, temporal, central, parietal, and occipital). Odd-numbered ones are on the left, and even-numbered ones on the right. The Pg electrodes attach to nasopharyngeal leads and the A electrodes, the ears (aural leads).
TABLE 10-1 Common Electroencephalogram Rhythms
| Activity | Hz (cycles/second) | Usual Location |
|---|---|---|
| Alpha | 8–13 | Posterior |
| Beta | > 13 | Anterior |
| Theta | 4–7 | Generalized* |
| Delta | < 4 | Generalized* |
The normal background rhythm in an awake adult consists of waves of activity in the alpha range of 8–13 cycles per second (Hertz [Hz]) detectable mostly over the occipital region (Fig. 10-2). Neurologists refer to this pattern as the posterior dominant rhythm. It is prominent when individuals are relaxed with their eyes closed, but disappears if they open their eyes, concentrate, or are apprehensive. When people undergoing an EEG merely fix their gaze on a clock or add two single-digit numbers, faster rhythms replace alpha activity. Preoccupations, concerns, or anxiety eliminate alpha activity. Because alpha activity reflects an anxiety-free state, it represents an important parameter in “alpha training,” biofeedback, and other behavior modification techniques.
FIGURE 10-2 A, Alpha rhythm consists of regular 8–13-Hz activity overlying the occipital lobe. B, Beta rhythm consists of low-voltage, irregular >13-Hz activity overlying the frontal lobe. C, Theta rhythm consists of 4–7-Hz activity overlying the right frontal lobe. D, Delta activity consists of high-voltage <4-Hz activity present over the entire hemisphere.
Theta (4–7 Hz) and delta (< 4 Hz) activities occur normally in children and everyone during deep sleep, but are usually absent in healthy alert adults. When present over the entire brain, theta or delta activity in wakefulness often indicates a neurodegenerative illness, such as Alzheimer disease, or a metabolic derangement. Continuous focal slow activity with phase reversal in bipolar montages (Fig. 10-3) sometimes originates in an underlying cerebral lesion; however, the absence of theta or delta activity certainly does not exclude one.
FIGURE 10-3 This bipolar montage shows four channels from the right side (upper four) and four from the left (lower four). Each side progresses from the frontal to the occipital region. On at least five occasions (marked by dots), sharp waves and spikes, in phase reversal, appear to point toward each other. These sharp waves and spikes originate from the common electrode, F3, situated over the left frontal lobe. Such isolated, phase-reversed sharp waves are associated with seizures, but, without additional clinical or EEG evidence, they are insufficient for a diagnosis of seizures.
Seizures
In some epilepsy patients, specially placed electrodes reveal abnormalities undetectable by ordinary scalp electrodes. For example, anterior temporal scalp, nasopharyngeal, or sphenoidal electrodes can detect discharges from the temporal lobe’s inferior-medial (mesial or medial) surface (Fig. 10-4).
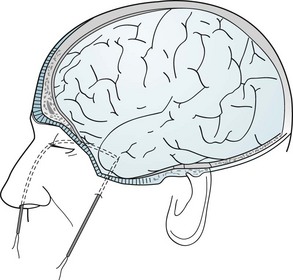
FIGURE 10-4 Nasopharyngeal electrodes, which are inserted through the nostrils, reach the posterior pharynx. There, separated by the thin sphenoid bone, they are adjacent to the temporal lobe’s medial surface, which is the focus (origin) of about 80% of complex partial seizures. (Figures in Chapter 20 show the relatively large distance between the temporal lobe’s medial surface and the scalp, and the closeness of the temporal lobe to the sphenoid bone.) Sphenoidal electrodes are inserted through the skin to reach the lateral surface of the sphenoid wing. Electrodes in this location are near the temporal lobe’s inferior surface. (Although nasopharyngeal and sphenoidal electrodes are valuable, specially placed scalp electrodes, new arrays, electronic filters, and critical reading of the EEG may be just as accurate and less invasive.) To pinpoint a seizure focus in anticipation of its surgical removal, neurosurgeons place a grid of electrodes in the subdural space.
With children, an evaluation could actually begin with parents making videos of suspected seizures or other episodic disturbances, including temper tantrums, breath-holding spells, night terrors, other parasomnias (see Chapter 17), dopamine-responsive dystonia, and other intermittent abnormal movements (see Chapter 18).
Toxic-Metabolic Encephalopathy
During the initial phase of toxic-metabolic encephalopathy (delirium), when patients have only subtle behavioral or cognitive disturbances, theta and delta activity replaces alpha activity. The organization of the EEG deteriorates as the patient’s sensorium disintegrates. The EEG in toxic-metabolic encephalopathy almost always shows generalized slowing and disorganization. Additional EEG changes point to specific diagnoses. Hepatic and uremic encephalopathies characteristically produce triphasic waves (Fig. 10-5). In fact, with hepatic failure, triphasic waves often appear before bilirubin levels rise. While metabolic derangements are the most common cause of triphasic waves, this EEG finding may also be seen with toxic levels of several medications, including lithium. Benzodiazepine use produces beta activity. Herpes simplex encephalitis produces spikes and periodic lateralizing epileptiform discharges over the temporal lobes.
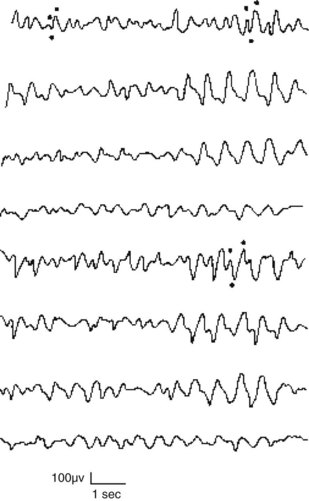
FIGURE 10-5 This EEG obtained from a patient with hepatic encephalopathy reveals characteristic triphasic waves overlying the frontal lobes (the first and fifth channels in this montage). In addition to the presence of triphasic waves, the EEG lacks the normal, organized alpha activity over the occipital lobes (the fourth and eighth channels).
Dementia
Vascular dementia also induces EEG abnormalities. However, these changes cannot reliably differentiate vascular dementia from Alzheimer disease dementia (see Chapters 7 and 11).
In contrast, the EEG is almost definitive in diagnosing subacute sclerosing panencephalitis and common, sporadic Creutzfeldt–Jakob disease (see Chapter 7). In these conditions – characterized clinically by dementia and myoclonus – the EEG shows periodic sharp-wave complexes (Fig. 10-6). (Variant Creutzfeldt–Jakob disease [“mad cow disease”] fails to produce these EEG changes [see Chapter 7].)
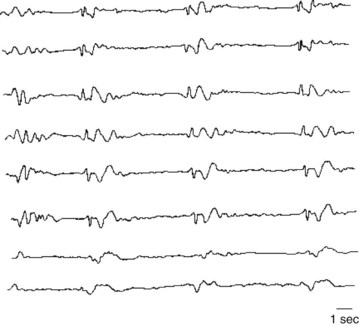
FIGURE 10-6 Periodic sharp-wave complexes classically appear in all channels as fairly regular, 1–3-second bursts of electrical activity followed by minimal activity. Neurologists often describe this EEG pattern as “burst suppression.” Myoclonic jerks represent the clinical counterpart of the periodic complexes. Together myoclonic jerks and periodic complexes are cardinal features of two illnesses characterized by dementia: subacute sclerosing panencephalitis and Creutzfeldt–Jakob disease (see Chapter 7).
The EEG can also help distinguish between pseudodementia and dementia – to the extent that they constitute separate entities (see Chapter 7). In pseudodementia the EEG ideally would remain normal, but in dementia from almost any cause, it would show slowing. For the many patients with a mixture of depression and mild dementia, the EEG cannot measure each condition’s relative contribution to cognitive impairment.
Structural Lesions
The EEG does not reliably detect or exclude structural lesions, even those that frequently cause seizures, such as brain tumors and cysticercosis. In fact, the EEG is normal in many of these conditions and, when abnormal, does not distinguish among them. Computed tomography (CT) and especially magnetic resonance imaging (MRI) are standard and, despite their expense, cost-effective for detecting structural lesions (see Chapter 20). Although CT may suffice, MRI better detects small structural lesions. More important, MRI is especially effective in detecting mesial temporal sclerosis, which underlies many complex partial seizures (see later).
Altered States of Awareness
The EEG shows distinctive changes during normal progressively deeper stages of sleep and during dreaming. Coupled with monitors of ocular movement and muscle activity in the polysomnogram, the EEG is critical in diagnosing sleep disturbances (see Chapter 17), which can include sleep-related behavioral disturbances and involuntary movement disorders, as well as seizures.
The EEG is also useful in diagnosing the locked-in syndrome, a condition in which patients cannot speak or move their trunk or limbs. Although patients in the locked-in syndrome appear comatose or demented, they remain fully alert and in possession of their cognitive capacity (see Chapter 11). The locked-in syndrome most commonly results from either an infarction in the base of the lower brainstem or extensive cranial and peripheral nerve damage. With their cerebral hemispheres and upper brainstem intact, patients retain normal cerebral activity and normal EEG activity.
Seizure Varieties
The two major seizure categories are partial (or focal)-onset seizures and primary (generalized) seizures. Most partial seizures are subclassified either as partial seizures with elementary symptoms or partial seizures with complex symptoms, and most generalized seizures are subclassified as either absence seizures (absences) or tonic-clonic seizures (Box 10-1).
Box 10-1
International Classification of Epilepsies by Seizure Type (Modified Version)
Elementary Partial Seizures
Partial seizures with elementary motor symptoms, formerly called focal motor seizures, typically consist of rhythmic jerking (clonic movement) of a body region that may be limited to one finger or extend to an entire side of the body (Fig. 10-7). These seizures may evolve into partial status epilepticus or undergo secondary generalization. Sometimes, in a “Jacksonian march,” a seizure discharge spreads along the motor cortex and creates movements, which are usually clonic, beginning in one finger and extending to the entire arm and then the face.
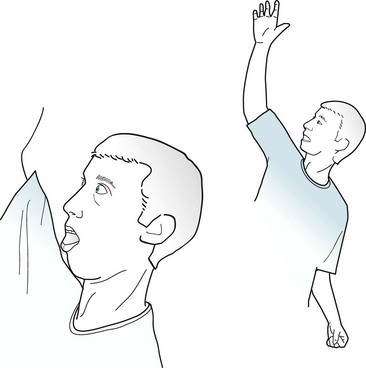
FIGURE 10-7 In this patient having a partial seizure with motor symptoms, his head, neck, and eyes deviate toward the right, his right arm extends, and his left arm flexes. A focal epileptic discharge in the contralateral left frontal lobe is producing this typical “adversive posture.”
Patients with auditory symptoms, which are attributable to temporal lobe lesions, frequently report hearing repetitive noises, musical notes, or single meaningless words. Visual symptoms, which are attributable to occipital lesions, usually consist of bright lights. Sometimes, though, these seizures may produce lines, spots, or splotches of color that move slowly across the visual field or, like a view through a kaleidoscope, rotate around the center of vision. Physicians must distinguish elaborate visual seizure phenomena from visual hallucinations due to other causes (see Box 12-1).
EEG and Etiology
During elementary partial seizures, EEGs show spikes, sharp or slow waves, or spike-wave complexes overlying the seizure focus. For example, during seizures with motor symptoms, EEG abnormalities are usually detectable in channels overlying the frontal lobe (Fig. 10-8), and, during the interictal period, EEGs may still show occasional spikes in the same channels.
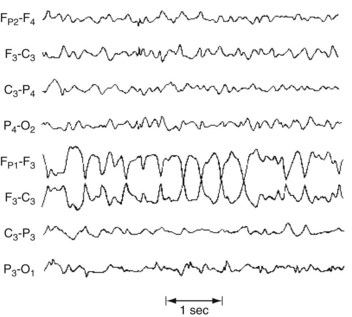
FIGURE 10-8 During a partial seizure with motor symptoms, this EEG contains a paroxysm of 4-Hz sharp-wave activity with phase reversals referable to the F3 electrode. Because the F3 electrode overlies the left frontal region, the seizure probably consists of right face or arm motor activity and, in some of the cases, a deviation of the head and eyes to the right.
In most cases, neurologists cannot determine the cause of partial seizures. Of those cases where neurologists can establish the cause, the patient’s age at the onset of the seizures is one of the most important factors. For example, when young children develop partial seizures, typical causes are congenital cerebral malformations, such as cortical dysgenesis, and neurocutaneous disorders (see Chapter 13). In young adults, common causes of elementary partial seizures are head trauma, arteriovenous malformations (AVMs), and previously asymptomatic congenital injuries. However, posttraumatic seizures are not associated with trivial head injuries, but with serious trauma, such as trauma causing more than 30 minutes of unconsciousness, depressed (not just linear) skull fractures, intracranial hematomas, and penetrating wounds.
Complex Partial Seizures
Ictal Symptoms
During most of a complex partial seizure, patients usually display a blank stare and are inattentive and uncommunicative. They always – by definition – have impaired consciousness. In most cases, they also have partial or complete memory loss, amnesia, presumably because seizure discharges besiege the limbic system in the temporal lobe. The amnesia is so striking that it may appear to be a patient’s only symptom. (Thus, physicians should strongly consider complex partial seizures among the neurologic causes of the acute amnestic syndrome [see Box 7-1].)
Physical manifestations of complex partial seizures usually consist of only simple, repetitive, purposeless movements (automatisms) of the face and hands. Present in about 80% of complex partial seizures originating in the temporal lobe, common automatisms include repetitive swallowing, mouthing, kissing, lip smacking, and lip licking – oral automatisms – or fumbling with clothing, scratching, rubbing the abdomen, or fidgeting – manual automatisms (Fig. 10-9). Other physical manifestations are simple actions, such as standing, walking, pacing, or even driving; however, sometimes these actions are simply ingrained tasks that continue despite the seizure. In addition, more than 25% of patients utter brief phrases or unintelligible sounds.
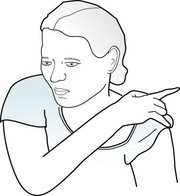
FIGURE 10-9 During complex partial seizures, patients typically appear dazed. They perform only rudimentary, purposeless actions, such as pulling on their clothing, and pay little or no attention to their surroundings or examiners. Their hands and fingers move in clumsy and misdirected patterns. Repetitive, simple oral, limb, or body movements, automatisms, such as lip smacking, occur in approximately 80% of cases with a temporal lobe focus and the majority of those with absence seizures.
Sex, Violence, and Aggression
During seizures, patients sometimes fumble with buttons, tug at their clothing, or make rudimentary masturbatory movements. They may even seem to undress partially. However, these patients are not deliberately exposing themselves or attempting to engage in sex. Except for very rare instances, seizures are unaccompanied by erotic or interactive sexual behavior. In fact, most seizure-like symptoms that develop during sexual activity, such as lightheadedness, are simply manifestations of anxiety. (On the other hand, severe headaches that develop during sexual intercourse are ominous [see coital headache and subarachnoid hemorrhage, Chapter 9].)
Immediate Postictal Symptoms
Immediately after a complex partial seizure, which has an average duration of 2–3 minutes, patients characteristically experience confusion, clouding of the sensorium, disorientation, flat affect, and sleepiness. However, seizures occasionally lead not to somnolence, inactivity, and withdrawal, but to agitation, i.e., postictal agitation. If seizures involve the brain’s language region and cause transient aphasia (see Chapter 8), postictal symptoms may be more pronounced. Similarly, if the seizure focus includes the cortical areas involved with motor function, patients may have a Todd’s hemiparesis. For 15–40 minutes after a seizure, many patients have measurable physiologic changes: Approximately 40% of them have an elevated serum prolactin concentration and focal EEG depression.
Frontal Lobe Seizures
Frontal lobe seizures, even compared to complex partial seizures of temporal lobe origin, are most apt to cause bizarre behavior and the few instances of aggressive violence. With their behavioral manifestations and EEG changes usually undetectable, frontal lobe seizures mimic PNES. In addition, when frontal lobe seizures arise exclusively during sleep, they mimic sleep disorders (see Chapter 17).
Testing During and Between Complex Partial Seizures
EEG
During a complex partial seizure, the EEG most often shows paroxysms of spikes, slow waves, or other abnormalities in channels overlying the temporal or frontotemporal region. Even though a seizure focus may be unilateral, bilateral EEG abnormalities appear because of additional foci, interhemispheric projections, or “reflections.” Nasopharyngeal and other specially placed leads may capture temporal lobe discharges that routine scalp electrodes fail to detect (Fig. 10-10).
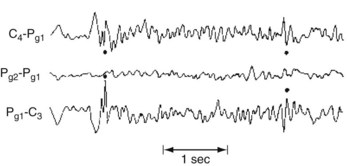
FIGURE 10-10 An interictal EEG with nasopharyngeal electrodes (Pg1 and Pg2) shows phase-reversed spikes that routine scalp electrodes may not detect.
Other Tests
Because elementary partial and complex partial seizures usually originate from structural lesions, neurologists routinely order MRIs unless patients have a contraindication (see Chapter 20). With even greater resolution than CT and freedom from artifacts produced by the bones surrounding the middle fossa, MRI reveals mesial temporal lobe sclerosis, tuberous sclerosis nodules, small strokes, and cryptic AVMs, as well as overt lesions (see Fig. 20-26). (MRI with thin cuts through the temporal lobes is often required to detect mesial temporal lobe sclerosis.)
Comorbid Conditions and Their Treatment
Cognitive Impairment
Of individuals with either a congenital intellectual disability (which the preliminary version of the DSM-5 calls Intellectual Developmental Disorder, but neurologists persist in calling “mental retardation”) or cerebral palsy, 10–20% have comorbid epilepsy (see Chapter 13). In them, epilepsy usually appears before age 5 years and its incidence increases in proportion to their physical and intellectual impairments. Of individuals institutionalized because of these disorders, 40% have epilepsy. Also, children with autism and, more so, Rett syndrome are susceptible to seizures (see before).
Destructive Behavior
Suicide
A landmark 2008 meta-analysis of clinical trials involving AEDs suggested an iatrogenic component. It found that, among epilepsy patients, AEDs represented almost a twofold risk factor for “suicidality” (suicide acts or ideation). Suicidality was greater in patients taking AEDs for epilepsy than for other indications, including mood stabilization, and among individuals taking multiple AEDs rather than a single AED. Subsequent studies found that only certain AEDs, such as those associated with causing depressive symptoms, placed epilepsy patients at risk. In sharp contrast to the 2008 study, Arana et al. (see References), in an equally credible one, determined that AEDs posed no risk of suicidality in patients with epilepsy.
Crime and Interictal Violence
Personality Traits
Classic studies, such as those by Bear and Fedio (see References), described “temporal lobe epilepsy” patients as distinctively circumstantial in thinking, hyposexual, humorless, “sticky” in interpersonal relations, and overly concerned with general philosophic and religious questions. These patients showed excessive and compulsive writing (hypergraphia). Supporting studies suggested that the presence of these abnormal traits depended on whether the seizure focus was in the right or left temporal lobe. Right-sided foci supposedly predisposed patients to anger, sadness, and elation, but left-sided ones to ruminative and intellectual tendencies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








