Fig. 35.1
Shortly after falling asleep, spikes-and-wave paroxysm are starting in a patient with CSWS. CSWS continuous spike-waves during slow sleep
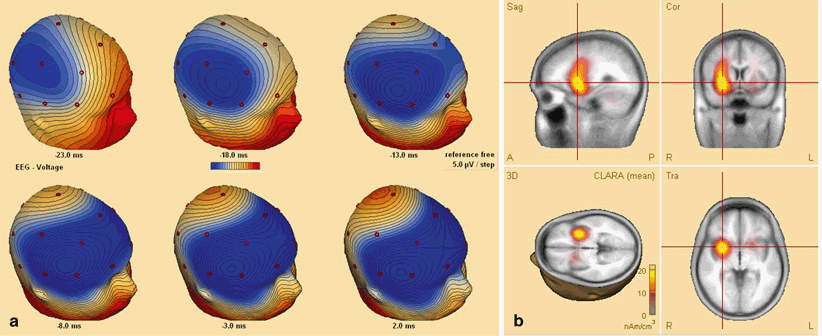
Fig. 35.2
Secondary bilateral synchrony in a patient with CSWS. a Sequential 3-dimensional voltage map on the ascending slope of the averages spike. The colour code indicates the polarity and the amplitude; negative potential is in blue, positive potentials are in red. Notice that at the onset, the negativity is only in the right central region; then it propagates bilaterally to the frontal region. b Source localisation (distributed source model, using BESA software) at the onset of the spike, suggesting that the initial electric activation is a focal one, localised in the right insula. CSWS continuous spike-waves during slow sleep, BESA brain electrical source analysis
The neuropsychological impairment ranges from language-related impairment, temporo-spatial disorientation to global cognitive decline with marked impairment of IQ, mental retardation, attention deficit and behavioural changes, aggressiveness, and difficulty in contact [47].
As with the other syndromes related to this type of epileptic encephalopathy , the course is favourable concerning seizures and the EEG pattern. Complete control of seizures is usually achieved within one year. Patients respond well to valproate and benzodiazepines . The EEG pattern (ESES) disappears within 3 years. Steroids might help in suppressing the nocturnal paroxysmal EEG activity and improve the neuropsychological functions. In spite of disappearance of seizures and ESES, and the improvement of cognitive functions, the prognosis is guarded: Less than half of the patients manage to live a normal life [47].
Idiopathic Generalised Epilepsies
IGEs represent a group of syndromes, comprising childhood and juvenile absence epilepsies, juvenile myoclonic epilepsy, and epilepsy with generalised tonic–clonic seizures on awakening. They are considered “awakening” type of epilepsies (Fig. 35.3; [19]). However, some patients have rare, nocturnal seizures.
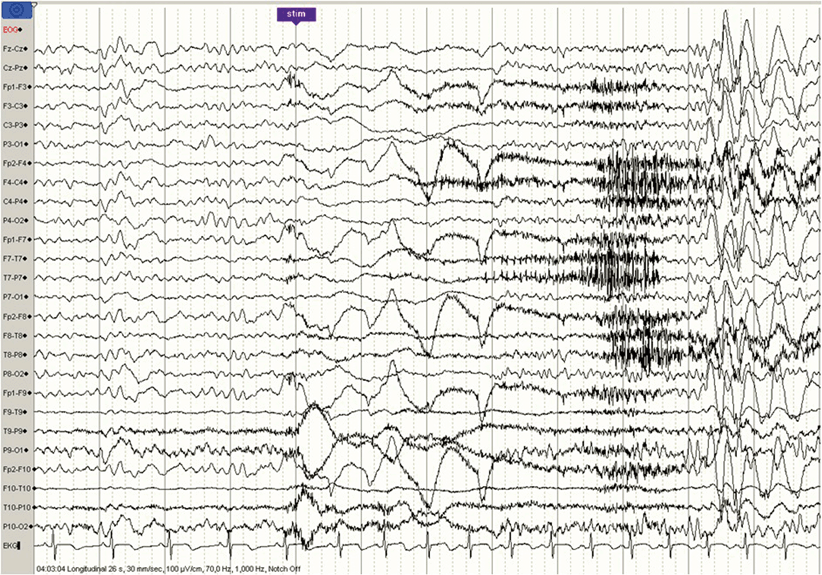

Fig. 35.3
Effect of awakening on the epileptiform activity in a patient with JME: shortly (6 s) after awakening, bilateral, fronto-central paroxysms are triggered. JME juvenile myoclonic epilepsy
Generalised tonic–clonic seizures, when occurring during sleep, start from stage 2 or during a shift between sleep stages, probably precipitated by a sudden clinical or abortive awakening; they never occur during REM sleep.
Myoclonic seizures can occur during sleep, and this imposes sometimes a differential-diagnostic problem. Periodic paroxysmal leg movements do not have EEG correlate, whilst epileptic myoclonias ictally are associated with polyspike-wave discharges.
Electrographic patterns corresponding to classical, “generalised” spike-and-slow-wave complexes are often recorded during sleep, though with reduction in its frequency. During sleep, it is impossible to assess the major clinical manifestation (“absence”); however, discrete eyelid myoclonia are often noticed time locked to the discharges.
Patients with awakening epilepsies have low-sleep efficiency. Sleep deprivation is an important provocative factor for awakening epilepsies, but it does not seem to affect the sleep epilepsies.
Other Epilepsies
In Lennox–Gastaut syndrome, the 10-Hz run-of-rapid spikes are precipitated during non-REM sleep, and are associated with tonic seizures of variable intensity (from discrete, only detectable by co-registering surface EMG signals, to major, generalised tonic seizures).
Patients with ring chromosome 20 have subtle, nocturnal seizures, often resembling awakening.
Several other seizure types occur less often during sleep than in wakefulness: myoclonia in patients with Dravet syndrome, seizures in the late childhood occipital epilepsy (Gastaut-type), and symptomatic parietal and occipital lobe epilepsies.
Effect of Epilepsy and of Antiepileptic Medication on Sleep
Patients with epilepsy often complain about disturbed sleep and excessive daytime drowsiness [50]. This is partly due to the effect of antiepileptic medication, but epilepsy itself affects the sleep structure, especially REM sleep [51].
Nocturnal seizures , especially the temporal seizures cause awakening, and the long postictal period (seen often) interferes with sleep. Nocturnal temporal lobe seizures cause decrease in REM sleep and sleep efficiency [51]. Furthermore, patients with TLE have decreased sleep efficiency also in the seizure-free periods.
As sleep disorders are common in the general population, therefore, these may occur coincidentally in patients with epilepsy [51]. This generates a vicious circle: Sleep disruption worsens epilepsy , which, in turn, worsens the sleep disorder.
A little appreciated aspect of sleep is its being a phase in the circadian rhythm . However, significantly contrasting circadian patterns of sleep and activity in JME versus TLE have been reported by Pung and Schmitz [52].
In a series of investigations with newly diagnosed patients comparing unmedicated baseline sleep with phenobarbital, phenytoin, ethosuximide, and valproate [53], ethosuximide was the only substance that had a major impact on sleep structure producing a marked decrease of depth of sleep with increase of stage 1 sleep. This corresponded well with frequent complaints of patients on this drug about sleep disturbances. Phenobarbital increased stage 2 sleep and decreased REM sleep; in awakening epilepsies, but not in sleep epilepsies, it decreased transitional states around REM phases which correlates with its preferential therapeutic effect in these epilepsies that have their spike maxima here [53]. A longitudinal evaluation of phenytoin as part of this project showed that initial effects largely disappeared over time, and the individual sleep pattern was mostly restored [54].
Likewise, several generally positive initial effects of carbamazepine on sleep have been described but seemed to disappear over time [55]. Whereas vigabatrine seems to have no significant effect on sleep, improvement is noted with gabapentin and clonazepam , the latter though producing an increase in spindle density [55]. Lamotrigine (LTG) was reported to increase REM percentage and decrease slow-wave sleep and the number of stage shifts [56]. This study on 13 patients seems not to have included patients who developed insomnia and delayed sleep onset, otherwise a not infrequent, sometimes rather annoying side effect of LTG. Little is yet known about possible influences of still newer AEDs on sleep.
As a caveat, improvements of sleep with administration of AEDs may be the indirect consequence of the therapeutic effect with reduction or disappearance of sleep-disturbing seizures or epileptiform discharges during sleep.
Conclusions
EEG is the most important investigation for both sleep and epilepsy , and sleep is the most important provocative tool for EEG in the diagnosis and differential diagnosis of epilepsy. Video observation (with and without EEG) may not only be necessary to distinguish epileptic seizures from other sleep-related disorders like parasomnias but also from normal sleep-related motor phenomena. These can be quite similar, especially when they use identical, sometimes complex, phylogenetically old innate motor patterns.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





