Fig. 1
Trajectory of patient performance on the CRS-R across a 10-week course of inpatient rehabilitation. Panel A shows the change in total CRS-R scores over time. During weeks 1 and 2, scores consistently remained in the vegetative range. In week 3, the total score sharply increased and then slowly transitioned into the MCS range during weeks 4 through 6. Although variable, the total score progressively improved through week 8 and was near ceiling, signaling emergence from MCS. Panel B compares recovery curves across the six CRS-R subscales. In week 3, a marked disparity emerged between motor (triangles) and language (diamonds, x’s and asterisks) performance. Despite the absence of any evidence of language comprehension or expression during weeks 4–6 (Auditory subscale scores <3; Oromotor-Verbal subscale scores <3; Communication subscale scores <2), there was clear and consistent evidence of complex automatic motor behavior (Motor subscale scores >4), including picking up objects and returning social gestures. This pattern of findings raised the possibility that an underlying aphasia may have been contributing to the absence of command-following and expressive speech. Following week 7, reproducible command-following was noted (Auditory subscale = 3), providing some evidence of language comprehension. During week 8, functional object use was demonstrated indicating emergence from MCS, however, command-following remained inconsistent, yes–no communication attempts remained unreliable and paraphasic verbalizations were noted. Taken together, the early dissociation between language and motor performance was likely related to an underlying aphasia
National Institute on Neurologic Disorders and Stroke—TBI CDE: www.commondataelements.ninds.nih.gov
Center for Outcome Measurement in Brain Injury: www.COMBI.org
Spaulding-Harvard TBI Model System Program: www.SH-TBIMS.org
A training DVD is available by request at the following website sponsored by the Coma Science Group: www.coma.ulg.ac.be
(iii) Recommendations for Use in Clinical Care. The CRS-R has been widely used in both clinical practice and research. In the clinical domain, common indications include differential diagnosis (e.g., Is the patient in a vegetative or MCS?), establishing prognosis (e.g., Is the rate of recovery above or below average for patients with DOC?), gauging functional outcome (e.g., Is the patient able to communicate reliably?), treatment planning (e.g., Are yes–no responses consistent enough to support use of an augmentative communication system?), and assessing response to treatment (e.g., Did the frequency/accuracy of command-following improve following introduction of a neurostimulant?). In research applications, the CRS-R has served as a prognostic indicator in outcome prediction studies [2, 7], as an outcome measure in clinical trials [10, 11], and as a reference standard in diagnostic neuroimaging and electrophysiology validation studies [12–14].
The CRS-R is the only standardized neurobehavioral assessment instrument that directly incorporates all of the diagnostic criteria required to distinguish VS from MCS, and MCS from emergence from MCS [6]. There is evidence that this feature improves diagnostic sensitivity as the scale has been shown to outperform clinician consensus in detecting signs of MCS [7].
Serial CRS-R assessments may also alert the examiner to subclinical changes in medical status. For example, a sharp decrease in the total CRS-R score may signal the onset of occult illness. We have observed situations in which the total CRS-R score has precipitously declined by more than five points prior to detection of systemic infection. Conversely, we have had cases in which a chronically low CRS-R total score (i.e., <10) demonstrates a sharp increase after initiating antibiotics for suspected respiratory illness. The rate of recovery, as reflected by the change in the total CRS-R score over a 4-week period (e.g., week 4–week 1), can also help predict outcome and disposition needs (e.g., outpatient program, inpatient rehabilitation hospital, skilled nursing facility).
(iv) Key Research. Psychometric Properties: The psychometric properties of the CRS-R have been extensively studied by investigators in the U.S. and Europe. In their 2004 standardization study, Giacino and colleagues administered the CRS-R to 80 patients, all of whom were unable to follow commands or communicate reliably [2]. Inter-rater reliability was high for the total CRS-R score, high for the auditory, motor, oromotor/verbal, and communication subscale scores, and moderate for the visual subscale. Similarly, test-retest reliability was high for the total score, and for the auditory, motor, communication, and visual subscale scores. Tests of internal consistency showed a significant relationship between the total CRS-R score and individual subscale scores, demonstrating the measure’s homogeneity. Subscale inter-correlations were moderate with the exception of a low inter-correlation between the visual and oromotor/verbal subscale. The CRS-R also showed adequate concurrent validity with the original version of the CRS and with the DRS. In a separate analysis of 20 subjects focusing on diagnostic agreement, inter-rater agreement for diagnosis was consistent in 16 of 20 cases assessed by two different examiners on the same day, and diagnosis remained stable across two examinations completed by the same examiner on consecutive days (i.e., test-retest reliability) in 18 out of 20 cases.
In 2010, an expert panel was convened by the American Congress of Rehabilitation Medicine to conduct a systematic review of the literature on the psychometric properties of behavioral rating scales designed for patients with DOC [6]. The review found the standardized administration, scoring and interpretive guidelines user-friendly and the psychometric properties (i.e., construct validity, internal consistency, inter-rater reliability, test-retest reliability) robust. The CRS-R was the only measure of the 13 scales examined to be recommended with “minor” reservations for use in patients with DOC.
Employing an Italian version of the CRS-R, Sacco and colleagues found good inter-rater and test-retest reliability for both total and subscale scores when experienced raters administered the scale [15]. A Norwegian study completed by Løvstad and colleagues explored the influence of rater experience on the reliability and diagnostic validity of the CRS-R. Raters from six different hospitals with three levels of experience established a diagnosis after independently administering and scoring the CRS-R twice over 3 days. Results again showed adequate reliability and validity; however, more experienced raters were more accurate in distinguishing VS and MCS than less experienced raters [8]. Inter-rater reliability for CRS-R scores was greater when highly experienced raters were paired with moderately experienced raters than when highly experienced raters were paired with newly trained raters. Test-retest reliability was greater among moderately experienced raters relative to newly trained raters, and there were no significant correlations in scores between the newly trained raters. Regarding diagnostic agreement, inter-rater and test-retest reliability were better among highly experienced raters than among less experienced raters (e.g., test-retest: 88 % for highly and moderately experienced raters vs. 50 % agreement between highly experienced and newly trained raters).
Schnakers et al. investigated the psychometric properties of a French translation of the CRS-R in a cohort of patients in VS and MCS [16]. Inter-rater and test-retest reliability were found to be satisfactory, and validity analyses showed significant correlations between total scores on the French CRS-R and scores on three other measures of level of consciousness—the Glasgow Coma Scale, Full Outline of UnResponsiveness, and Wessex Head Injury Matrix. The authors also found strong diagnostic agreement between raters. A fourth validation study conducted by Simões et al. using a Portuguese version of the CRS-R showed high inter-rater and test-retest reliability for both the total score and the six subscale scores [17].
A recently-published study by La Porta and colleagues in Italy applied Rasch analysis to further explore the measurement properties of the CRS-R [18]. Rasch analysis iteratively examines the underlying constructs assessed by rating scales and determines the “fit” between the individual items and the scale in its entirety. Scales with high degrees of “fit” are capable of interval measurement, allowing the examiner to gauge the distance between items using a measurement constant referred to as a “logit.” Within and between-subject comparisons can also be performed. La Porta, et al. collected CRS-R data from 129 participants across five hospitals using an Italian translation of the scale. Twelve experienced raters administered the CRS-R twice per patient extracting a total of 258 scores. Rasch analysis demonstrated excellent internal construct validity and satisfied all the principles required for interval measurement. The ordering of the scoring categories for the six subscales remained stable across different settings and raters with variable levels of experience, and scores were invariant regardless of the length of time post-injury, setting, age, or sex of the patients. The authors concluded that the CRS-R provides a linear measure of ability and, thus, is appropriate for use at the level of the individual patient.
Clinical Applications
Differential Diagnosis
Giacino et al. compared the CRS-R to the DRS in differentiating MCS from VS in a subsample of subjects included in their 2004 standardization study [2]. While the rate of diagnostic agreement between the CRS-R and the DRS was 87.5 %, the CRS-R detected evidence of conscious awareness in 10 cases (of 80) in which the DRS yielded a diagnosis of VS. Most of the cases missed by the DRS demonstrated evidence of visual pursuit on the CRS-R, a key diagnostic criterion not investigated by the DRS.
In 2009, Schnakers et al. compared diagnoses established by the CRS-R with those made based on clinical consensus of the rehabilitation team [7]. Data were collected from 103 patients with mixed injury etiologies who were followed by specialized neurorehabilitation teams. The investigators reported that 41 % of patients diagnosed with VS by team consensus (via qualitative observational assessment) had at least one sign of conscious awareness based on CRS-R examination. Similarly, 10 % of patients diagnosed with MCS by team consensus met criteria for emergence from MCS when examined with the CRS-R. Failure to detect purposeful eye movements accounted for most of the cases missed by simple observational assessment.
The CRS-R includes an unscored supplementary scale designed to detect behaviors that occur selectively in response to specific stimuli as this type of contingent relationship between a triggering stimulus and a specific response is associated with conscious awareness. Formisano et al. introduced the Post-Coma Scale (PCS) to incorporate emotional responsiveness into the assessment of patients with DOC [19]. The PCS seeks to supplement the assessment of features of MCS by incorporating a measure of emotional responsiveness. In this study, patients were diagnosed based on scores on the CRS-R and the PCS, both of which were administered by a single professional or by a professional in the presence of a caregiver. Results showed a significant positive correlation between PCS and CRS-R scores. Of note, patients at higher levels of consciousness demonstrated more emotional responsiveness. In addition, patients scored higher on both the CRS-R and PCS when caregivers were present during the exam, suggesting that the presence of caregivers may induce sufficient emotional stimulation to drive volitional behaviors.
Outcome Assessment
The CRS-R can be used to assess outcome either by monitoring “difference scores” (i.e., change in total score from time 1 to time 2) or by tracking changes in diagnosis (e.g., transition from VS to MCS or MCS to confusional state). Diagnosis has been shown to be of prognostic importance, as patients in MCS generally have more favorable outcomes than those in VS after controlling for chronicity. Katz and coworkers retrospectively examined the recovery trajectories of 36 patients diagnosed with VS or MCS on the CRS-R at admission to rehabilitation [20]. Patients were evaluated across the inpatient rehabilitation course and followed at least once between 1 and 4 years post-injury. Of the 11 patients admitted with a diagnosis of VS, 8 transitioned to MCS after spending an average of 8 weeks in VS. Approximately 70 % of patients admitted to rehabilitation in either VS or MCS emerged from MCS. However, when patients were segregated by admitting diagnosis (VS vs. MCS), almost twice as many patients in MCS emerged (80 %) relative to those in VS (45 %).
Noé et al. prospectively followed 32 patients with DOC on the CRS-R for at least 6 months or until emergence from MCS [21]. Approximately 25 % of the sample emerged from MCS within 6 months of injury. The rate of emergence was higher in the group admitted in MCS (35 % vs. 8 %). MCS subjects who did not emerge after the 6 month follow-up period (n = 13) were reassessed an average of 15 months after injury. No significant changes in neurological status were observed in any of the subjects followed.
Estraneo and colleagues employed the CRS-R to prospectively monitor outcome in 50 patients who remained in VS for at least 6 months after severe acquired brain injury. Patients were followed for an average of 26 months after injury [22]. At the final follow-up, 24 % had transitioned to MCS and, of these, 20 % recovered consciousness after 12 months post-injury. Six of the 10 patients who recovered consciousness late did so between 18 and 26 months post-injury. Of note, four patients in non-traumatic VS (three with anoxia and one with hemorrhagic stroke) recovered signs of consciousness on the CRS-R well beyond the 3-month cutoff established for “permanent VS” by the Multi-Society Task Force on PVS [23].
Monitoring Treatment Effectiveness
The standardized administration format and quantitative approach to assessment championed by the CRS-R suggest it is a suitable instrument for monitoring the effectiveness of treatment interventions applied in patients with DOC. The CRS-R has been used to monitor rate of recovery in patients diagnosed with VS and MCS who were exposed to amantadine hydrochloride vs. placebo [10] and to detect changes in alertness, motor function, and communication ability in a patient with chronic posttraumatic MCS following deep brain stimulation of the central thalamus [11].
The CRS-R has also been used in program evaluation. Seel et al. employed the CRS-R to investigate the effectiveness of a specialized early neurorehabilitation program for patients with DOC [24]. The investigators found that patients improved from admission to discharge on all six subscales of the CRS-R and 53 % had emerged from MCS by discharge.
Use of the CRS-R as a Reference Standard
In view of the absence of a gold standard for detection of conscious awareness, investigators have employed the CRS-R as a “reference standard” to compare the results of neuroimaging and electrophysiologic, behavioral studies. Rodriguez-Moreno and colleagues developed an fMRI paradigm in which ten subjects diagnosed with VS, MCS, EMCS, or Locked-In Syndrome (LIS) were shown pictures of common objects and instructed to silently name each object presented [14]. The authors found that the degree of activation of the language network observed during the task correlated with the subjects’ CRS-R score, and that activation patterns in subjects who attained high CRS-R scores (i.e., LIS, EMCS) approximated healthy controls while those with low CRS-R scores showed little to no activation.
Similarly, a series of recent studies have shown that CRS-R total scores correlate with metabolic activity in critical cortical networks [25–27]. Total CRS-R scores have been found to be higher in patients who retain activity in frontoparietal midline structures thought to mediate internal, stimulus-independent “self” consciousness [25, 28], while scores on the Auditory, Oromotor-Verbal and Communication subscales have been shown to be lower in patients with metabolic dysfunction in the dominant language hemisphere [26] and with white matter connectivity measured with diffusion tensor imaging [29].
Bekinschtein and others explored the relationship between level of consciousness and electromyographic (EMG) changes in response to movement commands presented to ten patients diagnosed with VS or MCS on the CRS-R [30]. Subjects received verbal commands to move their right or left hands or to remain still while EMG and video recordings monitored spontaneous muscle activity. Two patients (one MCS and one VS) who failed to produce any observable motor activity showed significantly more EMG activity following administration of the movement command than when instructed to simply rest. These findings suggest that bedside EMG monitoring may improve diagnostic accuracy in patients who evidence little to no active movement.
Electrophsiologic studies employing the CRS-R have shown that measures of EEG entropy in the left frontotemporal region correlate with CRS-R scores obtained in the acute but not chronic stage of recovery [12] and with resting state EEG measures of cortical connectivity [31].
Recent experimental studies have demonstrated an association between cortical excitability induced by transcranial magnetic stimulation (TMS) and level of consciousness gauged by the CRS-R. Lapitskaya and colleagues found a significant correlation between short-latency afferent inhibition values and CRS-R scores in 47 patients with DOC (24 VS and 23 MCS) and 14 healthy controls [32]. Similarly, Casali et al. found a graded relationship between CRS-R total scores and the duration and complexity of EEG activity induced by TMS pulses in patients diagnosed with coma, VS, MCS, emergence from MCS, and LIS [33]. Using the CRS-R as a benchmark, the investigators were able to distinguish all subjects who were alert from those who were completely unconscious based on the complexity of the EEG signal.
Confusion Assessment Protocol [34]
(i) Background and Purpose. TBI is synonymous with disordered consciousness. Persons with severe injuries have a period of complete loss of consciousness called coma that persists for hours after the injury. Persons who survive severe TBI generally recover to a state of, at least, partial consciousness, though a few remain in an unconscious, vegetative state. Persons with moderate injuries have briefer periods of unconsciousness, and have, by definition, recovered partial consciousness by the time of presentation to the emergency department. Persons with mild TBI often have no period of complete loss of consciousness, but rather have a period of altered consciousness that is characterized by inability to form and later recall new memories. For patients who have recovered the ability to respond to the environment in a relatively consistent, meaningful way, the next period of recovery is characterized by confusion [35]. Some refer to this confusional state as delirium and yet others use the term posttraumatic encephalopathy. We prefer the term posttraumatic confusional state (PTCS). While PTCS is a subtype of delirium, delirium can be caused by a wide range of medical conditions ranging from substance withdrawal to end-stage organ failure. The courses, prognoses, and likely underlying neuropathologies of these diverse conditions vary greatly making delirium an imprecise term [36]. Posttraumatic encephalopathy is a similarly imprecise term as encephalopathy refers to any disease or disruption of brain function.
Our research and clinical experience indicate that PTCS is likely ubiquitous in moderate and severe TBI. Persons with mild TBI may not show a full confusional state though many do. PTCS is characterized by disorientation, cognitive impairment, fluctuation in presentation, agitation, decreased daytime arousal, nighttime sleep disturbance, and psychotic-type symptoms [34]. Our research has shown that assessment of PTCS informs judgments about long-term prognosis and that PTCS is characterized by a particular pattern of recovery with decreased daytime arousal, nighttime sleep disturbance, and psychotic-type symptoms recovering earliest post-injury and fluctuation and cognitive impairment persisting longest post-injury [37].
PTCS has clear implications for early clinical management including rehabilitation. Confused patients pose increased risk of injury to self and others. Confusional states are associated with increased duration and cost of care [38]. Confused patients have poorer cooperation with therapy activities. Family members/significant others are distressed to see their loved ones confused and may over-interpret words and actions that flow from this confusion. The Confusion Assessment Protocol (CAP) [34] was developed to provide a standardized procedure for diagnosing persons as confused or non-confused and to facilitate serial tracking of patient recovery over time. The CAP was designed to be easy to administer, score, and interpret.
(ii) Administration and Scoring. The CAP is a collection of scales and items developed by a number of TBI researchers. These scales and items were selected for inclusion in the CAP based on their ability to distinguish patients who met diagnostic criteria for delirium for those who did not and based on coverage of the seven key symptoms of PTCS identified by the authors of the CAP. In developing the CAP, new scoring rules were created for some of the items, some items were modified, and a methodology was created for combining scores from items to determine whether a patient is confused or not.
Cognitive impairment and orientation are measured with performance measures. Cognitive impairment is measured by items taken from the Cognitive Test of Delirium [39] and the Toronto Test of Acute Recovery after TBI [35]. These items measure basic attentional and mental control functions. Patients are asked to count to 20 forwards and backwards and recite the months forwards and backwards. On a simple vigilance task, patients indicate when a target letter is spoken and inhibit any response to non-target letters. Patients also answer simple reasoning questions. Finally, patients are shown five pictures that they must identify from a field of ten pictures after a delay. In selecting these items, the authors found that more demanding tasks such as word list learning were failed by virtually all persons in early recovery from TBI so that such items did not assist in distinguishing confused patients from non-confused patients.
Orientation is assessed using the Galveston Orientation and Amnesia Test [40]. This test requires the patient to give personal information (name, date of birth, residence), current circumstances (current location, date of admission to the hospital, means of conveyance to the hospital), first memory after injury, last memory before injury, time, day, and date.
Agitation, fluctuation, nighttime sleep disturbance, decreased daytime arousal, and psychotic-type symptoms are rated using scales or items selected from scales. Ratings for each of these symptoms are based on observations over a 24 h period. The rater should base these scores on his/her own interactions with the patient as well as reports from nurses, therapists, family members, and others are appropriate. Agitation is measured with the Agitated Behavior Scale [41]. Fluctuation and psychotic-type symptoms are measured using items from the Delirium Rating Scale-R-98 [42]. An item from the Delirium Rating Scale-R-98 was modified to measure nighttime sleep disturbance and a new item was created to assess decreased daytime arousal.
Once measures are administered and scales rated, performances are classified as consistent with confusion or not consistent with confusion based on scoring criteria developed by the authors of the CAP. Persons with four or more symptoms consistent with confusion are in PTCS and persons with three symptoms consistent with confusion are in PTCS if one of these symptoms is disorientation. All others are not in PTCS. Confused patients with three or four symptoms of confusion are in mild confusion, those with five symptoms are in moderate confusion, and those with six or seven symptoms are in severe confusion. CAP forms and other information can be downloaded at http://www.tbims.org/combi/cap/index.html and additional information on the CAP can be obtained by contacting Mark Sherer at Mark.Sherer@memorialhermann.org.
(iii) Recommendations for Use in Clinical Care. On the inpatient neurorehabilitation unit, we recommend that all patients with TBI who are not in vegetative or MCSs at admission are assessed with the CAP on the day after admission. We find that some patients are so fatigued by the transfer process that findings from assessments on the day of admission may be misleading. The CAP should be implemented with initially vegetative or minimally conscious patients once they recover to a responsive state. Once begun, CAP assessments should continue on a two or three times a week basis until confusion resolves. Confusion is considered to have resolved when the patient obtains non-confused scores on two consecutive CAP assessments that are at least 24 h apart. Some may prefer to require three consecutive non-confused CAPs as we have seen some patients perform in the confused range on subsequent evaluations even after obtaining two consecutive non-confused scores. Of course, some patients may regress due to factors such as seizures, posttraumatic hydrocephalus, medication effects, sleep disturbance, etc. Patients who receive non-confused scores on the initial two CAPs are considered non-confused at admission and no other CAP examination need be performed. Absent medical complications or medication effects, patients are expected to show a recovering course for symptoms of confusion. We have found that roughly 75 % percent of patients show a decreased number of symptoms of confusion from the first to the third CAP assessment covering a period of 4–5 days. Failure to improve should prompt an assessment for possible treatable problems that are interfering with recovery.
CAP results can be reported in the medical records and patient staffings by reporting confusion status (confused, not confused), number of symptoms of confusion present, GOAT score, ABS score, cognitive impairment score, etc. Graphic presentation of results over time is helpful to the rehabilitation team, physicians, and family/significant others. Note that some patients who have just emerged from MCS at the initiation of CAP assessment may show increasing numbers of symptoms of confusion as they become more responsive and exhibit more behavior overall. However, most patients will show a recovering course with decreasing numbers of symptoms and even those who begin assessment early after emergence from MCS will eventually show a recovering course. Posttraumatic confusion is a transitional phase of recovery and patients will not remain confused indefinitely. A few patients may remain so amnestic that they never recover orientation, but other symptoms of confusion such as sleep disturbance, psychotic symptoms, decreased daytime arousal, agitation, and fluctuation will continue to resolve so that even the disoriented patient with severe or profound cognitive impairment will usually eventually emerge from confusion based on CAP diagnostic criteria. As with patients with dementia, patients with TBI who have persistent, severe cognitive impairment are at increased risk of becoming confused when stressed by medical conditions, medication effects, pain, sleep disturbance, or other factors.
(iv) Key Research. The CAP has been validated by comparing CAP classifications of patients as confused vs. non-confused to clinical diagnosis of delirium, showing that CAP findings are predictive of functional status outcomes both early after injury and at 1 year follow-up, demonstrating the associations of CAP scores with an important rehabilitation issue—cooperation with treatment, and illustrating patterns of recovery of symptoms of confusion. These studies provide strong support for the CAP as a research and clinical instrument. However, to this point, all studies published on the CAP have been conducted by the research group that originally developed the CAP. Sometimes assessment instruments do not perform comparably when used by groups other than the original group that developed the instrument. Clinicians and researchers will have more confidence in the CAP if findings shown by Sherer and colleagues are cross-validated by research conducted by other groups.
Sherer and colleagues [34] described the development of the CAP and presented early findings. The researchers selected CAP items and developed scoring rules based on the ability of these items to distinguish TBI patients who were clinically diagnosed as being in delirium using DSM IV criteria from those who were not in delirium. CAP development was based on findings from 62 persons with TBI who were admitted to a neurorehabilitation unit. In the same paper, the authors provided some initial validation data for the CAP in a new series of 112 patients with TBI who were studied during inpatient brain injury rehabilitation. Combining the CAP development sample with initial validation sample yields a group of 174 persons with TBI. For this combined group, CAP diagnosis of confusion was 93 % sensitive and 86 % specific for a clinical diagnosis of delirium indicating very good agreement. Sherer and colleagues have also provided data on the phenomenology of PTCS based on their seven key symptoms of confusion. As shown in Fig. 2, fluctuation in presentation was the most common symptom occurring in all confused patients with cognitive impairment occurring in about 95 % and disorientation occurring in over 90 % of confused patients. Psychotic-type symptoms were the least frequent symptom occurring in about 45 % of confused patients. There was overlap in symptoms between confused and non-confused patients for all symptoms other than disorientation which did not occur in non-confused patients. Decreased daytime arousal, restlessness (agitation), and psychotic-type symptoms were all rare in non-confused patients with each occurring in no more than 10 % of cases. Fluctuation and cognitive impairment were relatively common in non-confused patients who were in early recovery from TBI occurring in about 45 % and 35 % of patients, respectively. Multivariable linear regression analysis based on 80 cases showed that even after adjustment for other predictors including initial injury severity (days to follow commands), confusion status at rehabilitation admission predicted functional status at rehabilitation discharge with patients who were in PTCS at admission having poorer functional status.
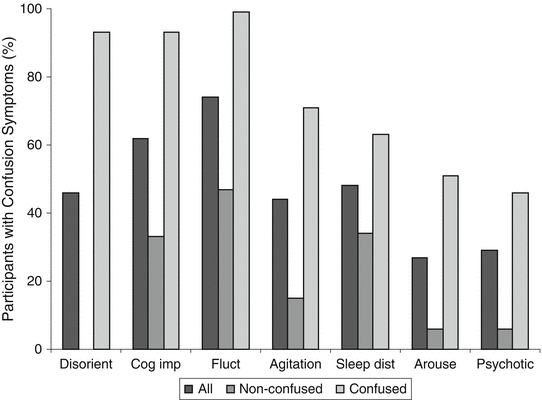

Fig. 2
Phenomenology of confusion in patients with traumatic brain injury. Disorient indicates Disorientation, Cog imp—Cognitive impairment, Fluct—Fluctuation, Sleep dist—Nighttime sleep disturbance, Arouse—Decreased daytime arousal, Psychotic—Psychotic-type symptoms. Adapted with permission from Sherer M, Yablon SA, Nakase-Richardson R, Nick T. Effect of severity of posttraumatic confusion and its constituent symptoms on outcome after traumatic brain injury. Arch Phys Med Rehabil 2008;89:42–47
Sherer and colleagues [43] extended earlier findings by showing that CAP data were not only predictive of functional status at rehabilitation discharge, but also of productivity outcome at 1 year post-injury. Reporting on a series of 168 persons with TBI admitted for inpatient rehabilitation, Sherer and colleagues showed that number of symptoms of confusion (possible range 0–7) shown on a single CAP evaluation at about 21 days post-injury was predictive of functional status at discharge from inpatient rehabilitation and of productivity status at 1 year post-injury. After adjustment for other predictors including injury severity (initial Glasgow Coma Scale score and days to follow commands), patients with only two symptoms of confusion at 21 days post-injury were about three times as likely to have favorable functional status at discharge as compared to those with five symptoms of confusion. Further, patients with two symptoms at 21 days post-injury were twice as likely to be productive (competitively employed or in school) at late follow-up 1 year post-injury as compared to those with five symptoms. An exploratory analysis of the significance of each individual symptom of confusion showed that absence of each symptom of confusion was associated with more favorable overall outcome than presence of each symptom. In these analyses, simple odds ratios were calculated and there was no adjustment for other predictors. A surprising finding was that patients who did not show psychotic symptoms at 21 days post-injury were over 14 times more likely to be productive at 1 year follow-up than patients who did show psychotic-type symptoms. This finding was obtained even though no patients in this series showed onset of a new psychotic disorder meaning that psychotic symptoms in all patients resolved when confusion resolved.
Sherer and colleagues [37] provided additional data on patterns of symptom recovery in confused patients. In a series of 107 confused patients assessed during inpatient neurorehabilitation, patients were generally shown to have rapid recovery of symptoms. From the first CAP assessment to the second CAP assessment (about 2 days), over 50 % of patients showed a decrease in the number of symptoms of confusion and from the second CAP to the third CAP (again about 2 days) over 50 % of patients with initial moderate or severe confusion again showed a decrease in the number of symptoms. Only about 25 % of patients with initial mild confusion showed a decrease in number of symptoms due to a floor effect. Data showed that nighttime sleep disturbance, decreased daytime arousal, and psychotic-type symptoms are the earliest symptoms of confusion to resolve while fluctuation and cognitive impairment are the most persistent symptoms of confusion.
Silva and colleagues [44] examined the relationship between posttraumatic confusion as measured by the CAP with cooperation with treatment for persons with TBI undergoing inpatient rehabilitation. A series of 74 patients with TBI undergoing inpatient rehabilitation were assessed three times weekly with the CAP, while therapists treating the patients rated the degree of cooperation with therapy. CAP scores and therapist ratings were obtained independently. A regression model including only the number of CAP symptoms accounted for 25 % of the variability in cooperation ratings, while the full model including age at injury, years of education completed, time since injury, and injury severity (Glasgow Coma Scale score) accounted for 33 % of the variability in cooperation. Greater confusion was associated with poorer cooperation. To examine the associations of specific symptoms of confusion with cooperation ratings, Spearman’s coefficient was calculated. Decreased daytime arousal showed the strongest association with a Spearman’s coefficient of −0.42. Correlations for restlessness (agitation), psychotic symptoms, and cognitive impairment were −0.39, −0.39, and −0.24, respectively. In each case, presence of the symptom was associated with poorer cooperation.
Finally, Sherer and colleagues [45] followed up on earlier findings regarding the significance of psychotic symptoms in confused patients. In a series of 107 patients with TBI in inpatient rehabilitation who completed a total of 640 CAP assessments, Sherer and colleagues examined factors that were associated with the occurrence of psychotic-type symptoms. The strongest association was found for nighttime sleep disturbance. Patients with nighttime sleep disturbance were over four times as likely to show psychotic-type symptoms as those without sleep disturbance. Patients were more likely to show psychotic-type symptoms early after rehabilitation admission rather than later after rehabilitation admission though time since injury did not predict psychotic symptoms. Patients with a greater degree of cognitive impairment were more likely to show psychotic symptoms than those with more intact cognitive functioning. Sherer and colleagues also replicated their earlier finding showing that presence of psychotic-type symptoms was associated with a decreased likelihood of a productive outcome at 1 year post-injury. However, in this analysis with adjustment for age, years of education, and injury severity (time to follow commands), the effect was not as great with those with no psychotic symptoms being about four times as likely to be productive as compared to 14 times more likely in the earlier unadjusted analysis.
Mississippi Aphasia Screening Test [46]
(i) Background and Purpose. Aphasia is a primary disturbance of the ability to use language. Aphasia can affect all aspects of language including expressive language, fluency of speech, repetition, naming, and language comprehension in both auditory and written modalities. As with other neurocognitive impairments, structured testing is essential in evaluating language functions as even experienced clinicians may under-estimate the degree of impairment if they rely on conversation or other non-structured interactions. For aphasics with preserved speech prosody and superficial social greetings, even very substantial language comprehension deficits may be missed. Language impairments are strongly associated with focal left hemisphere brain lesions, but can also be seen in patients with diffuse lesions such as those that often occur in persons with TBI. Absent a focal lesion in the language cortex, language impairment in persons with TBI tends to resolve as early confusion resolves and persistent aphasia after non-penetrating TBI is rare. Nonetheless, assessment of patients in early recovery after TBI should include assessment of language. It is critical to determine whether a patient can consistently and accurately respond yes and no to simple questions such as, “Are you in pain?” Yes/no responding is often the medium for patients to express preferences such as food choices in early recovery. Similarly, it is crucial to have an assessment of the patient’s ability to comprehend spoken and written language. Some patients may adequately comprehend short passages of language, but breakdown in their comprehension of longer passages. While this phenomenon may indicate impairment of attentional functions or memory rather than a primary language disorder, this information is still important to clinicians providing care to the patient and family members interacting with the patient.
Comprehensive aphasia batteries may require up to 2 h to administer. Such lengthy assessments may not be well tolerated by confused patients or other patients in early recovery from TBI. Since language impairment is unlikely to be the key deficit in persons with TBI due to blunt head trauma, detailed assessment with a comprehensive battery may not be warranted. A brief battery that assesses a broad range of language functions and can be repeated to document improvements in functioning is adequate for many patients. The Mississippi Aphasia Screening Test (MAST) is a brief, easily repeated battery that assesses key aspects of language function and has been used with persons with TBI.
(ii) Administration and Scoring. The MAST consists of nine subtests that assess (1) naming, (2) automatic speech, (3) repetition, (4) yes/no accuracy, (5) object recognition, (6) verbal command following, (7) written command following, (8) verbal fluency, and (9) writing to dictation. Each subtest generates a score and subtest scores range from 0 to 10 except for yes/no accuracy which ranges from 0 to 20. Subtest scores contribute to index scores for receptive and expressive language that each range from 0 to 50 and an overall score that can range from 0 to 100. As with many aphasia tests, the MAST is a test of impairment so that persons with no language impairment are expected to obtain perfect or near perfect scores. The entire test can be administered in 5–10 min.
For the naming subtest, the patient is asked to name five common objects (e.g., pen, hand, watch). For the automatic speech subtest, the patient is asked to count to ten, say the days of the week, and complete three sentences (e.g., Three strikes and you’re ___). On the repetition subtest, the patient repeats words and phrases (e.g., carrot, under the old wooden bridge). Verbal fluency is assessed by showing the patient a standard stimulus picture and asking the patient to describe what he/she sees. The number of items verbalized by the patient determines the score. If the patient can provide 11 or more verbalizations, he/she will receive the highest possible score of 10. For the naming, automatic speech, repetition, and writing to dictation subtests, each correct response is worth two points. The naming, automatic speech, repetition, verbal fluency, and writing to dictation scores are summed to generate the expressive language index score.
For the yes/no accuracy subtest, patients respond yes or no to ten questions ranging from “Is your name [patient’s correct]?” to “Does summer come after spring?” For the object recognition subtest, the subject is asked to point to a specific object from a field of five common objects. For verbal command following, the patient is asked to follow five verbal instructions and for written command following, the patient is asked to follow the same five instructions that are provided typed on sheets of paper. Each correct response for yes/no accuracy, object recognition, verbal command following, and written command following is worth two points. Yes/no accuracy, object recognition, verbal command following, and written command following scores are summed to calculate the receptive language index score and the expressive and receptive language index scores are totaled to yield the total score.
As noted above, with the possible exception of the verbal fluency subtest, neurotypical adults are expected to achieve essentially perfect scores. Any score less than perfect should be investigated. It is important to consider factors other than language impairment that can affect performance on the MAST. Patients in early recovery may have decreased daytime arousal and fluctuating attention. These factors can cause item failures even in those with intact language functions. Some patients may give poor effort because they believe the items are too simple and they are offended by being asked to complete such simple tasks. Other patients may refuse to complete the MAST altogether. Such non-compliance might reflect a general rejection of assessments, a rejection of assessments of cognitive abilities, or transitory fatigue or poor mood. Sometimes, refusals can be caused by some degree of self-awareness of deficits that motivates the patient to reject assessments so that he/she will not be confronted with deficits due to TBI.
It should be noted that non-patients reported on by Nakase-Thompson and colleagues [46] produced an average score substantially below the maximum score on the verbal fluency subtest, suggesting that scoring rules for the verbal fluency subtest may be too stringent. MAST users should be cautious in interpreting scores from this subtest.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





