CHAPTER 19
Evoked Potentials
I. Evoked Potentials (EPs)
A. Brainstem auditory-evoked responses (BAERs)
Classification of BAERs According to Latency
TYPE | LATENCY (MSECS) | PRESUMED SOURCE |
Early (short latency) | <12 |
|
Electrocochleogram | 1–4 | Auditory nerve compound AP |
BAERs | 1–12 | Wave I = auditory nerve AP |
|
| Waves II–V brainstem |
Middle | 12–50 | Myogenic vs. neurogenic source |
Transient |
|
|
Steady state |
|
|
Slow or late | >50 |
|
Late | 50–250 | Cortical (N100, P150, N200) |
Long | >250 | Cortical (P300) |
1. Early auditory-evoked potentials (AEPs)
a. Electrocochleogram
i. Sound waves travel via the external auditory canal to the tympanic membrane, in which they produce changes in air pressure and displacement of the tympanic membrane → displacements of the tympanic membrane are transmitted via the ossicular chain (malleus, incus, and stapes) to the oval window of the cochlea.
ii. The cochlea contains the cochlear duct, an endolymphatic epithelial tube; the endolymph is suspended within another space, the perilymphatic space, which is a spiral tube enclosed at one end by the footplate of the stapes in the oval window and at the other end by the round window; it is continuous with the vestibular labyrinth and cerebrospinal fluid; within the cochlear duct are the basilar membrane and organ of Corti; vibration or displacement of the stapedial foot plate causes change in perilymphatic pressure; vibrations transmitted at the stapedial foot plate are transmitted into a traveling wave at the basilar membrane; high-frequency vibes produce maximum displacement at the base of the cochlea, whereas low-frequency vibes produce maximum displacement at the apex; organ of Corti contains sensory cells: inner and outer hair cells that carry cilia of graded length, with longest embedded in tectorial membrane; when the basilar membrane vibrates, hair cell cilia bend against the tectorial membrane and are moved by the endolymphatic displacement, which produces electrical depolarization of hair cells (receptor potential); inner hair cells transmit to afferent nerve fibers of spiral ganglion cells (the first-order afferents of the auditory system) and from there form the cochlear nerve; a separate set of spiral ganglion fibers innervates the outer hair cells.
iii. Occurs within 2.5 milliseconds of stimulus
2. BAERs
a. Physiology
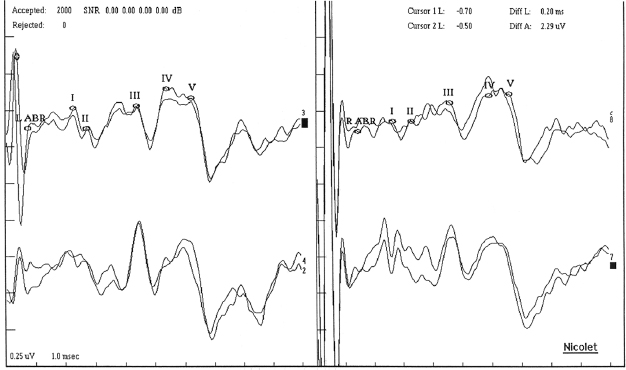
Figure 19.1 Brainstem auditory-evoked potentials. Wave legend: I, acoustic nerve; II, compound AP of the auditory nerve at the entrance into the brainstem, or cochlear nuclei (medulla); III, cochlear nucleus and trapezoid body or superior olivary complex (pons); IV, lateral lemniscus (pons) or superior olivary complex; V, inferior colliculus (midbrain).
Origin of BAER Waves
WAVE | GENERATOR/SOURCE |
I | Compound AP recorded from the distal end of the acoustic nerve or graded potential of dendritic terminals of the acoustic nerve; approximately 2 msecs positive stimulus |
II | Changes in current flow at the acusticus internus, or compound AP of the auditory nerve at the entrance into the brainstem, or graded potentials from cochlear nucleus |
III | Cochlear nucleus and trapezoid body or superior olivary complex and trapezoid body |
IV | Lateral lemniscus, ventral lemniscus cells, or superior olivary complex or ascending auditory fibers in the pons |
V | Generated by projections from the pons to the midbrain, including the ventrolateral inferior colliculus and ventrolateral lemniscus; approximately 6 msecs positive stimulus; first wave whose falling edge goes below baseline; last wave to disappear as stimulus intensity is dropped |
VI, VII | Higher brainstem structures, including thalamus (medial geniculate body) |
i. All waves used for assessment are negative potentials.
b. Recording and stimulus parameters
i. Earphones must completely envelop the ears to reduce ambient noise; for infants and young children, tubes are placed in the auditory canal because headphones may collapse the external canals.
ii. Three types of sounds are produced:
(A) Clicks: most frequently used for routine testing; produced by square wave pulse with the rising phase moving the diaphragm in one direction and the fall of the phase returning it to the origin; condensation = initial movement of diaphragm toward eardrum, refraction = away from eardrum (refraction is used predominantly); duration approximately 100 microseconds (producing a sound complex approximately 2 milliseconds in duration)
(B) Pure tone: delivers exact frequency; most commonly used for pure-tone audiometry to test hearing
(C) White noise: composed of all audible frequencies; delivered into nonstimulated ear to mask ambient noise and avoid bone conduction of the click to the contralateral ear
iii. Stimulus rates: 8 to 10 seconds (waves I, II, VI, and VII have reduced amplitudes at higher frequencies)
iv. Each acoustic stimulus can be broken down to three components:
(A) Frequency (Hertz): relates to the location of physical stimulation along the basilar membrane of the cochlea and along the tonotopic representation of the central auditory pathways
(B) Intensity (decibel): refers to the loudness of the stimulus
(C) Time: includes duration, rise–fall time, repetition rate, and phase of onset of the stimulus; the phase of onset refers to the initial direction of the basilar membrane displacement.
v. Intensity of an acoustic stimulus is measured in three ways:
(A) Hearing level: average threshold in decibels measured in normal adults (0-dB hearing level); ≈30 dB per sound pressure level (SPL)
(B) Sensation level: subject’s individual threshold (decibel sensation level)
(C) SPL: acoustic stimuli are measured in decibels peak equivalent SPL (dB SPL); SPL uses as a standard reference level of 20 micropascals.
vi. Recording electrodes should be placed over vertex and bilateral ears and/or bilateral mastoids.
vii. BAERs are relatively independent of level of consciousness and affected little by sedatives.
viii. Refraction clicks are recommended because patients with high-frequency hearing loss may have cancellation of out-of-phase responses by condensation and refraction.
ix. BAER latencies decrease in a quasilinear pattern with increasing stimulus intensities; waves I and V latencies increase as intensity decreases, whereas the I–V interpeak interval remains essentially unchanged; normative values for latency-intensity functions have been derived allowing for comparative analysis.
x. Age is another negative variable:
(A) In those born prematurely, waves II, IV, and VI are less well defined than I and V, and the I–V interpeak interval is longer than in adults.
(B) Latencies reach adult level by age 1 year.
(C) Latencies also increase as age increases, but the I–V interval usually remains the same; most of the changes are likely related to wave I as a result of associated cochlear dysfunction.
xi. Gender: females with shorter latencies (presumably attributable to body and brain size)
c. Clinical applications
i. BAERs are useful for:
(A) Hearing assessment in infants
(B) Assessing hearing loss in uncooperative adult
(C) Evaluating hearing in functional deafness
(D) Evaluating brainstem function
(1) Possible multiple sclerosis (MS)
(2) In central pontine myelinolysis: prolonged waves I–V and III–V latencies (damage to pontine region) with normal wave I (peripheral cochlear nerve/nucleus input)
(E) Evaluation of neuro-otologic disorders
(1) Acoustic neuromas
(2) Cerebellopontine angle tumors
(3) Brainstem lesions
ii. Interpretation usually requires measurement of waves I, II, and V and also I–III and I–V interpeak intervals.
iii. Use of latency-intensity functions allows differentiation of four types of pathology:
(A) Latency-intensity functions indicating conductive hearing loss: prolonged waves I and V with latency-intensity curves parallel to the normal curve; I–III and I–V intervals are normal.
(B) Latency-intensity functions indicating cochlear hearing loss: associated with high-frequency hearing loss; recruiting curve for wave I (i.e., normal or mildly prolonged wave I latencies with loud clicks and greater delays decreased intensity, resulting in a steep curve); wave V not markedly affected, and this curve less steep, resulting in a shortened I–V interval.
(C) Latency-intensity functions indicating retrocochlear deficit type I: wave I prolonged with steep latency-intensity function; wave V prolonged; therefore, I–V interval prolonged; associated with lesions of cranial nerve VIII.
(D) Latency-intensity functions indicating retrocochlear deficit type II: wave I latency-intensity curve is normal; wave V and I–V interval is prolonged.
iv. Prolonged I–V interpeak interval is most sensitive indicator of brainstem lesion; prolongation of the III–V interval alone suggests at or after the superior olivary complex (in either the high pons or low midbrain).
v. NB: Normal wave V latency practically rules out any peripheral or central lesions in the auditory pathway.
vi. Most common cause of impaired BAERs is demyelinating disease.
vii. ↑Age → ↑ hearing loss in high frequency (>1 kHz)
viii. In brain death, may have complete absence of BAERs or wave I and/or II (wave II noted in 10% of brain-dead patients, which reinforces theory that wave II is generated by intracranial portion of cranial nerve VIII)
ix. Important interpeak intervals may occur in metabolic derangements: vitamin B12 deficiency, meningitis, epilepsy, alcoholism, diabetes mellitus; diabetes mellitus and meningitis have findings consistent with damage to acoustic nerve.
x. Neurologic disorders that cause important transient BAERs
(A) Intramedullary brainstem tumors
(1) Wave I: usually preserved because acoustic nerve usually not involved
(2) Increased interpeak latency (IPL) I–III if pontomedullary
(3) Increased IPL III–V if pontomesencephalic or midbrain
(B) Cerebellopontine angle tumors
(1) Absence, increased latency, or increased duration of wave I, and subsequent waves are either distorted or absent but may be present and delayed.
(2) IPL I–III is often prolonged (if waves I and III are preserved), which is a sensitive indicator for cerebellopontine angle tumors.
(C) MS: no particular BAER impairment is specific for MS.
xi. General interpretation of BAERs
(A) Absent ipsilateral with contralateral normal: severe unilateral hearing loss due to unilateral cochlear or acoustic nerve lesion
(B) Absent bilaterally: bilateral acoustic nerve lesions, brain death; rule out technical problems.
(C) Absent wave I with normal III and V: peripheral hearing disorder with normal central conduction
(D) Absent peaks after normal wave I: ipsilateral proximal acoustic nerve, ipsilateral pontomedullary lesion
(E) Absent wave III with normal I and V: normal variant
(F) Absent wave V with normal I and III: ipsilateral lesion of brainstem (i.e., caudal pons)
(G) Low amplitude or prolonged latency of entire BAER bilaterally: peripheral hearing loss (especially conductive), distal acoustic nerve lesion; rule out reduced stimulus intensity; in distal acoustic nerve lesions, there may be prolonged latencies of entire BAERs but will have normal IPL I–V.
(H) Prolonged wave I latency and of all subsequent waves but normal IPL III–V: lesion of distal acoustic nerve, peripheral hearing loss
(I) Prolonged I–V IPL: most sensitive indicator of brainstem lesion
(J) Increased I–III IPL (but normal III–V): defect from between proximal acoustic nerve to inferior pons; most common impairment with acoustic neuromas
(K) Prolonged III–V IPL but normal I–III latencies: if only impaired, suggests lesion at or after the superior olivary complex in caudal pons or midbrain
(L) Increased I–III and III–V IPLs: IL lesion of lower and upper brainstem
(M) Increased BAERs threshold: suspect peripheral hearing loss; distal acoustic nerve lesion
(N) Parallel upward shift of latency-intensity curve: conductive hearing loss
(O) Shift of latency-intensity curves upward especially at low frequencies: sensorineural hearing loss
xii. Intraoperative BAERs monitoring
(A) During surgery in posterior and middle fossa
(B) Useful for acoustic nerve and brainstem surgery because BAERs are not affected by ordinary anesthetics (e.g., halothane, thiopental; the exceptions are enflurane and imipramine overdose, which increase IPLs)
(C) During acoustic neuroma surgery, the most common changes are loss of waves II–V or increased I–III IPL.
(D) Good but not perfect relation between deterioration of intraoperative BAERs and subsequent postoperative hearing deficits
xiii. Coma
(A) May help differentiate coma due to structural versus metabolic factors (because metabolic/toxic processes usually do not cause important BAERs unless irreversible structural damage is inflicted)
(B) Body temperature less than 32ºC may alter BAERs.
xiv. Conditions that may have normal BAERs: supratentorial lesions, spinocerebellar degeneration, Huntington’s chorea, vestibular neuronitis, Meniere’s disease, labyrinthitis
xv. BAERs in infants and children:
(A) All high-risk newborns (<1,500 g and infants in neonatal intensive care unit) should have BAERs within first month.
(B) If normal, nearly 100% will have normal hearing; if impaired, initiate rehabilitation, but be cautious because a small percentage will have significant hearing loss.
xvi. BAERs and audiometry
(A) Wave V is plotted versus stimulus intensities of 20, 40, 60, and 80 dB greater than hearing threshold, producing a semilog plot with an inverse linear relationship between intensity and latency in normal adults (↑ stimulus → ↑latency).
(B) Effect of hearing loss on threshold and latency
(1) Hearing loss increases threshold of BAERs, specifically that of wave V if the loss involves the frequencies 1 to 4 kHz, through which click stimuli exert their effect.
(2) Increases of threshold less than 30 dB above normal hearing threshold cannot be taken as important.
(3) Increases latency of wave V
(C) Conductive hearing loss
(1) Prolongs latency for all intensities, producing an upward shift of the curve but no change in slope
(2) Interferes with the conduction of sound waves from the ear canal to the cochlea; therefore, acts like a reduction of stimulus intensity that produces a lower amplitude and longer latency of all waves of the BAERs
(3) At low stimulus intensities, the latency of wave I is more increased than that of other waves in conductive hearing loss, so that IPL I–III and I–V are shortened.
(4) Does not apply to conductive hearing loss caused by ossicular chain disorders
(5) Impedance audiometry is just as effective as BAERs in assessment of conductive hearing loss.
(6) Latency-intensity curve
(a) Increases the latency of wave V over the range of all intensities, and therefore causes a parallel shift in the curve equivalent to the amount of hearing loss.
(b) The possibility of a central defect must be ruled out by determining that the I–V IPL is normal.
(D) Sensorineural hearing loss
(1) Produces a curve with two slopes; at low intensity, there is decreased responsiveness of end-organs so that for any given intensity → the latency is prolonged; with increased intensity, there is greater than normal recruitment of nerves, so that the curve is steeper; at exceedingly high intensities, there has been sufficient recruitment such that the latency may be normal with the remainder of the slope parallel to the slope of a normal patient (although usually shifted upward).
(2) BAER amplitude is reduced (at least at moderate stimulus intensities).
(3) Amplitude of ratio V to I is usually increased.
(4) Latency of wave V is increased (in keeping with the degree of hearing loss at 4 kHz).
(5) Wave I (if visible) is at least equally increased in latency, causing a normal or importantly short IPL I–V.
(6) Latency-intensity curve
(a) Greatest deviation from normal latency and amplitude at low stimulus intensities (the more the stimulus strength exceeds threshold, the less disparity between the normal and important curve; at high intensities, the latency may be normal, which causes the characteristic steepening of the latency-intensity curve becoming L-shaped)
3. Middle-latency AEPs
a. Occur 12 to 50 milliseconds after stimulation
b. Middle-latency AEPs uncontaminated by muscle potentials (older theory discusses myogenic etiology as source); probably include components of Heschl’s gyrus, thalamocortical projections, posterior temporal gyrus, angular gyrus, and the insula and claustrum
c. Lesions of the thalamus or midbrain are more likely to affect the middle-latency AEPs.
4. Late AEPs
a. Greater than 50 milliseconds postauditory stimulation
b. Subdivided into exogenous components N1, P1, and P2 that are primarily dependent on the external stimulus, and into endogenous components P300, N400, cranial nerve V (CNV), and the mismatch negativity (which are more dependent on internal cognitive processes)
c. Exogenous late AEPs are best elicited by tone bursts and have the highest amplitude over the vertex; N100 may be generated in a posterior-superior temporal plane and adjacent parietal cortex, whereas the later waves may arise from the auditory cortex and frontal association cortex.
d. N400 is a potential obtained to linguistic stimuli when there is semantic incongruity.
B. Somatosensory EPs (SSEPs)
1. Stimulation parameters
a. Unilateral stimulation of a motor/sensory nerve trunk sufficient to produce a moderate motor response is required.
b. Duration: stimulus artifact is reduced by shorter duration (approximately 100 microseconds); no more than 500 microseconds.
c. Rate: between 1 and 10 per second (faster than 10 per second is highly painful) and results in low-amplitude somatosensory response; faster rates prolong all absolute and interpeak latencies and suppress cortical amplitudes by 20% to 30% but produce little or no difference on subcortically generated potentials; thus, in short-latency (SSEPs) and AEPs, there is a direct relationship between rate and latency and an inverse relation between rate and amplitude.
d. Stimulators: either constant voltage or constant current
2. Recording: important aspect of recording EPs: replication of waveforms
3. Generators
a. Overview of stimulus and waveform patterns
i. Near field: cortically generated N20 is a near-field response with maximum voltage between the contralateral central and parietal electrodes on the scalp.
ii. Far field: in contrast, far-field component is generated by subcortical structures that reach the scalp relatively rapidly via conduction by fluid (e.g., cerebrospinal fluid) mediums (and not via neural paths); typically smaller (microvolts) and faster in frequency and latencies; topographic nonspecificity at scalp.
b. Most events of SSEPs are from dorsal column–lemniscal system (cuneate neurons not only project to contralateral thalamus, but also to other brainstem structures, including dorsal and medial accessory olives, portions of the inferior and superior colliculi, thalamic nuclei not in the specific projection system, etc.).
4. General clinical interpretation
a. The undiagnosed patient: owing to the nonspecific nature of SSEPs, they are of little clinical relevance for the already diagnosed patient, but may have substantial benefit in the undiagnosed patient.
b. MS
i. In patients with clinically definite MS, at least one of the EPs is positive in more than 75% to 90%; thus, if all EPs are normal, diagnosis of MS should be questioned.
ii. 33% to 50% of SSEPs: clinically silent lesions
iii. Diagnostic yield: visual EPs (VEPs) > SSEPs > BAERs
iv. MRI tends to be more sensitive than trimodality EP testing for evaluation of MS.
c. Peripheral nerve disorders
i. SSEPs are absent with severe peripheral nerve disease or lesion.
ii. May be useful notably to evaluate proximal nerve pathology (e.g., Guillain-Barré syndrome [GBS])
5. Median nerve SSEPs
a. Median nerve response components
i. Obligate waveforms
(A) Erb’s point potential (EP; N9 [or P9])
(1) Near-field triphasic (positive-negative-positive) potential; the prominent negative peak usually occurs at 9 milliseconds (N9) after stimulation at the wrist.
(2) Orthodromic sensory and antidromic motor APs ascending from peripheral nerve stimulation generate EP.
(3) The recording derivation includes negative input potential over the brachial plexus at Erb’s point (2 cm superior to the clavicular head of the sternocleidomastoid), and the positive input reference electrode is placed over the contralateral Erb’s point or shoulder.
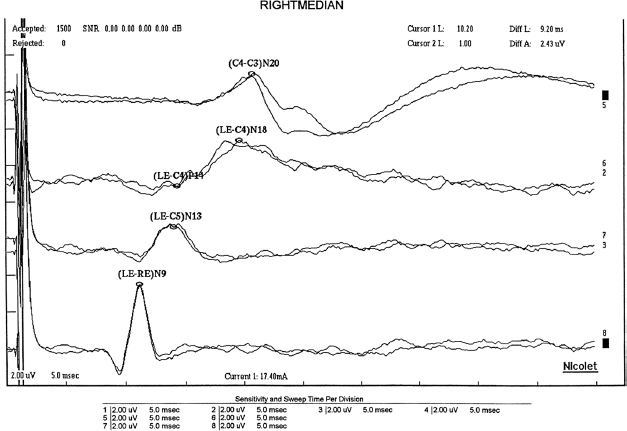
Figure 19.2 Median somatosensory-evoked potential.
(B) N13
(1) Near-field negative potential recorded over the dorsum of the neck (usually at 13 milliseconds after stimulation).
(2) Can be recorded referentially to any distal point, with Erb’s point being a good location because it provides in-phase cancellation of a superimposed slow potential seen at the neck and Erb’s point.
(3) Origin is dorsal horn neurons.
(C) P14
(1) A far-field positive peak present broadly over the scalp (approximately 14 milliseconds after stimulation)
(2) Subcortically generated and probably reflects activity in the dorsal column nuclei and/or the caudal medial lemniscus within the lower medulla
(3) In-phase cancellation if recorded scalp-to-scalp, and therefore, should be referenced to Erb’s point or torso
(D) N18
(1) Far-field relatively slow potential present broadly over the scalp
(2) Subcortically generated, probably from postsynaptic activity from multiple brainstem generators
(3) Inhalation anesthetics have little effect on N18 (as compared to N20).
(E) N20
(1) Near-field negative peak
(2) Generated by the primary cortical somatosensory receiving area
(3) When recorded referentially, the N20 is preceded by the far-field P9, P11, and P14 and is superimposed on the coincidental N18.
(4) Origin from thalamocortical radiations
(F) Late potentials
(1) Include P25, N30, P45
(2) Largely state dependent
(3) Typically, these are unsuitable for neuronal evaluation.
(4) Generators of these are believed to be associated with cortical association areas.
ii. Median nerve SSEP interpeak latencies
(A) EP-N20, EP-P14, P14-N20, and spinal lumbar potential (LP)-P37 (tibial SSEPs)
(B) Much more reliable than absolute latencies
(C) Eliminate most of the peripheral effects, as described earlier
(D) Not appreciably affected by age/gender in the adult population, but SSEPs of newborns differ substantially from adults because of the immaturity of myelination of the peripheral nervous system and CNS.
(1) In term neonates, median SSEPs reliably show subcortical potentials but do not demonstrate cortical response (N20) in more than one-third of neonates.
(2) Cortical response (N20) is not reliably seen until 2 to 3 months of age.
b. Clinical interpretation
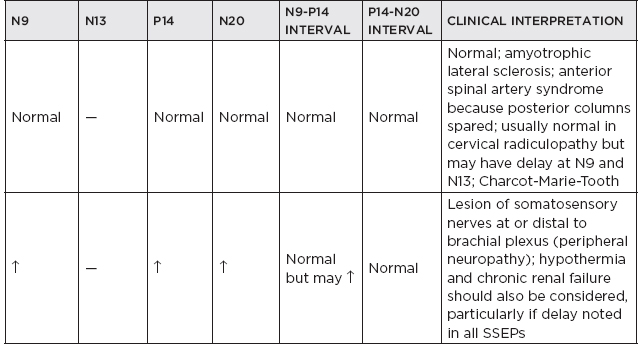
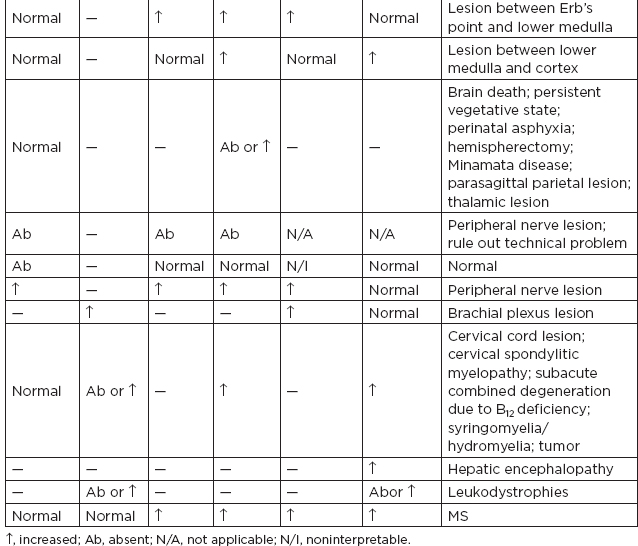
c. Brachial plexopathy
i. Criteria for impairment are decreases of 40% or more in amplitude of N13 or Erb’s point potential responses.
ii. If the N13 were absent or reduced to a greater extent than Erb’s point potential, then the lesion is more likely preganglionic.
iii. If the EP response was reduced to an equal or greater degree than N13, then the lesion is more likely postganglionic.
d. Radiculopathy/spondylosis/myelopathy
i. Radiculopathy without myelopathy: SSEPs are of little clinical benefit.
ii. Myelopathy due to cervical spondylosis.
(A) Usually seen and are attenuated or absent N13 and N20, a prolonged EP-N13 interval
(B) Usually little clinical benefit
iii. An increase in the clavicular-cervical (N9-N13) and the clavicular-scalp (N9-N20) conduction time combined with normal NCVs → is a reliable indicator of root involvement or cord involvement below the medulla.
iv. Cervical root lesions are characterized by preservation of the clavicular EP and of sensory nerve action potentials (SNAPs) (unless there is additional involvement of the brachial plexus).







