Fig. 1
Intracellular free zinc level in primary astrocytes. Primary astrocytes were treated with 0 or 100 μM zinc under 3 h hypoxia/12 h reoxygenation or 15 h normoxia condition. The intracellular free zinc was visualized using FluoZin-3 AM fluorescence probe
Hypoxia Decreased Extracellular Zinc
Obviously, hypoxia/reoxygenation increased endogenous free zinc level even when no zinc was added. However, what about that with zinc treatment? Is the increase of intracellular free zinc completely from endogenous zinc or is it also from exogenous zinc? We reasoned that if exogenous zinc uptake is a cause for the increase of intracellular free zinc, then extracellular zinc must decrease after hypoxia/reoxygenation treatment. Thus, following 100 μM zinc and the hypoxia/reoxygenation treatment, extracellular zinc was detected using a cell-impermeable zinc probe FluoZin-3 Cell Impermeant, which only stains zinc in the media. As shown in Fig. 2, just as we had predicted, the extracellular zinc decreased only after hypoxia/reoxygenation treatment, but not at normoxia, demonstrating that extracellular zinc uptake is, or at least is one of, the reasons for the increase in intracellular free zinc.
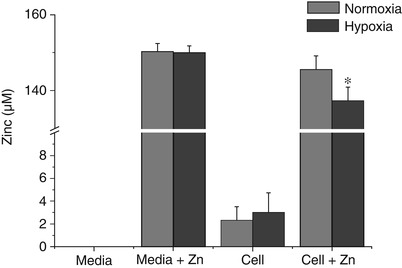

Fig. 2
Extracellular zinc level in the media. 100 μM zinc was added into the astrocytes culture media. After 3 h hypoxia/12 h reoxygenation or 15 h normoxia, zinc level in the media was measured by using FluoZin-3 Cell Impermeant probe. The experiments were repeated three times. Data were presented as mean ± standard error (SE). *p < 0.05 compared with the normoxia and zinc-treated group
Znt-1 Expression Reduced after Hypoxia/Reoxygenation Treatment
The regulation of cellular zinc homeostasis is controlled tightly through zinc transporters ZIP family and ZnTs [15]. By measuring the ZIP-1 expression, we found that hypoxia/reoxygenation hardly changes the ZIP-1 level in astrocytes. However, for the ZnT-1 protein, hypoxia/reoxygenation dramatically decreased ZnT-1 expression, which is difficult to reverse by zinc, although zinc significantly increased its expression in normoxic conditions. Remarkably, these results gave us an interesting suggestion: Compared with normoxic conditions, in the hypoxia/reoxygenation condition, zinc influx remained at a same level while the efflux of zinc was severely hampered. This may be one of the reasons why intracellular free zinc increases with extracellular zinc addition only in hypoxia/reoxygenation but not in normoxia.
Discussion
Evidence shows that intracellular zinc increases after ischemic stroke [12]. Excess intracellular free zinc has a well-documented role in contributing to cell injury [21–23]. Understanding the mechanism of intracellular free zinc accumulation is critically important for ischemic stroke research. The present study demonstrates a zinc efflux blocking function of hypoxia/reoxygenation. Under hypoxia/reoxygenation conditions, the zinc efflux mediator protein ZnT-1 level declined to less than half the level of that under normoxia conditions (Fig. 3). Moreover, although zinc caused a substantial jump in ZnT-1 level at normoxia, it was unable to reverse the loss of ZnT-1 caused by hypoxia/reoxygenation. However, in this situation, hypoxia did not change the zinc influx mediate protein ZIP-1 level. In addition, the loss of extracellular zinc (Fig. 2) provided more direct evidence for zinc influx greater than zinc efflux in hypoxic astrocytes. Our results demonstrate that hypoxia/reoxygenation decreased ZnT-1 protein level, preventing zinc efflux from cell, leading to increase of intercellular free zinc. It is the first time hypoxia/reoxygenation interrupting zinc homeostasis in astrocytes has been shown.
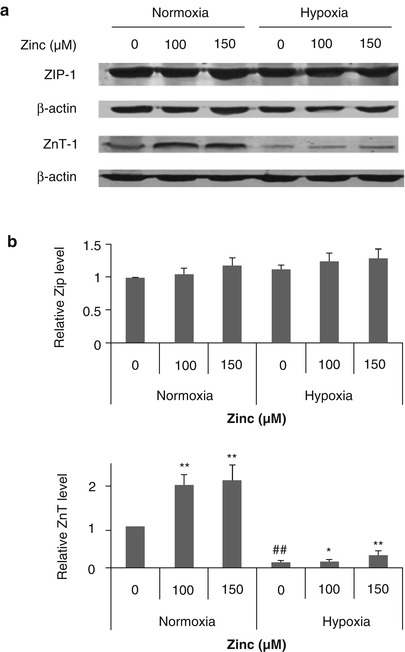

Fig. 3
The effect of zinc and hypoxia/reoxygenation on ZnT-1 and ZIP protein level. Astrocytes were treated with the indicated concentration of zinc under hypoxia/reoxygenation or normoxia. Representative immunoblots showing zinc transporter protein levels (a). Relative protein levels (b) were quantitated after normalization to β-actin. The experiments were repeated three times. Data were presented as mean ± SE. *p < 0.05, **p < 0.01 compared with the group without zinc treatment on each condition; ##p < 0.01 compared with normoxic astrocytes group
Zinc homeostasis in cells is considered to be tightly regulated by 10 members of ZnT (zinc transporter protein) and 14 members of ZIP (Zrt/IRT-like protein) families [24]. Among them, ZnT-1 mediates zinc efflux from cytoplasm to the extracellular space. Evidences show that ZnT-1 protein mediates zinc efflux, at the same time the expression of the ZnT-1 gene is also regulated by zinc. The reaction of ZnT-1 and zinc regulates intercellular zinc level [25]. Qin et al. [13] found that zinc was locked in cultured cortical neurons after ZnT-1 silence. Although, ZnT-1 mediation of zinc efflux is well documented, whether ZnT-1 contributes to intercellular free zinc accumulation in hypoxic cells had not been illustrated. Our results show that ZnT-1 was downregulated by hypoxia/reoxgenation, leading to zinc trapping in cell. Regarding zinc influx, the ZIP family is known to promote zinc influx to cytoplasm [15], however, there is no report on the relationship of ZIPs and intercellular free zinc accumulation in hypoxic astrocytes. ZIP-1, -2, and -4 transport zinc from extracellular space to cytoplasm [26]. Franklin et al. [27] reported that ZIP-1 is the major zinc uptake transporter for the accumulation of zinc. Thus, we only measured ZIP-1 level after zinc and hypoxia treatment. We found that the expression of ZIP-1 was similar in normoxic and hypoxic astrocytes. Our results indicate that in normoxic conditions, zinc influx and efflux were regulated by ZnT-1 and ZIP-1, keeping intracellular zinc at a normal and nontoxic concentration. Notably, in the hypoxia/reoxygenation condition, zinc influx remained at a normal level, whereas the loss of ZnT-1 disabled the zinc efflux. Thus, zinc was trapped in the cells, accumulating to a high and toxic concentration, which may be one of the reasons why ischemic stroke-induced zinc release causes brain injury. However, how hypoxia/reoxygenation affect the ZnT-1 protein needs further study.
In addition to exogenous zinc, endogenous zinc may also be a cause of intercellular zinc increase after ischemic stroke. The metallothioneins (MTs) protein family, functioning in absorption and temporary storage of zinc, is known to be involved in managing zinc cellular level [15, 28]. The bond between zinc and MT is tightly regulated by redox [29, 30], suggesting that after ischemic stroke, the intercellular free zinc increase may be from endogenous MT-bound zinc release. Moreover, the increase of intracellular free zinc without adding exogenous zinc also indicated that endogenous zinc is a reason for zinc accumulation in hypoxic astrocytes. To investigate whether exogenous zinc contributed to free zinc accumulation in addition to endogenous zinc, we added zinc to the cell culture media, and measured the remaining zinc in the media after hypoxia/reoxygenation or nomorxia treatment. We found that extracellular zinc only decreased in hypoxic astrocytes but not in normoxic astrocytes, indicating that the uptake of exogenous zinc is at least one of the reasons for intercellular free zinc increase.
In conclusion, our results demonstrate that hypoxia/reoxygenation significantly decreased ZnT-1 protein level, whereas it did not change ZIP-1 protein level, leading to intercellular free zinc accumulation. We found that hypoxia/reoxygenation decreased ZnT-1 level, while ZIP remained at a similar level as in normoxia. Our results demonstrate that hypoxia/reoxygenation blocked zinc efflux, whereas it had no effect on zinc influx, and that hypoxia/reoxygenation interrupted zinc homeostasis in astrocytes by limiting zinc return to extracellular space, providing a novel way for intracellular free zinc accumulation after ischemic stroke. These findings provide a novel mechanism accounting for intercellular free zinc accumulation after ischemic stroke.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (P20RR15636, P30GM103400, and R01AG031725).









