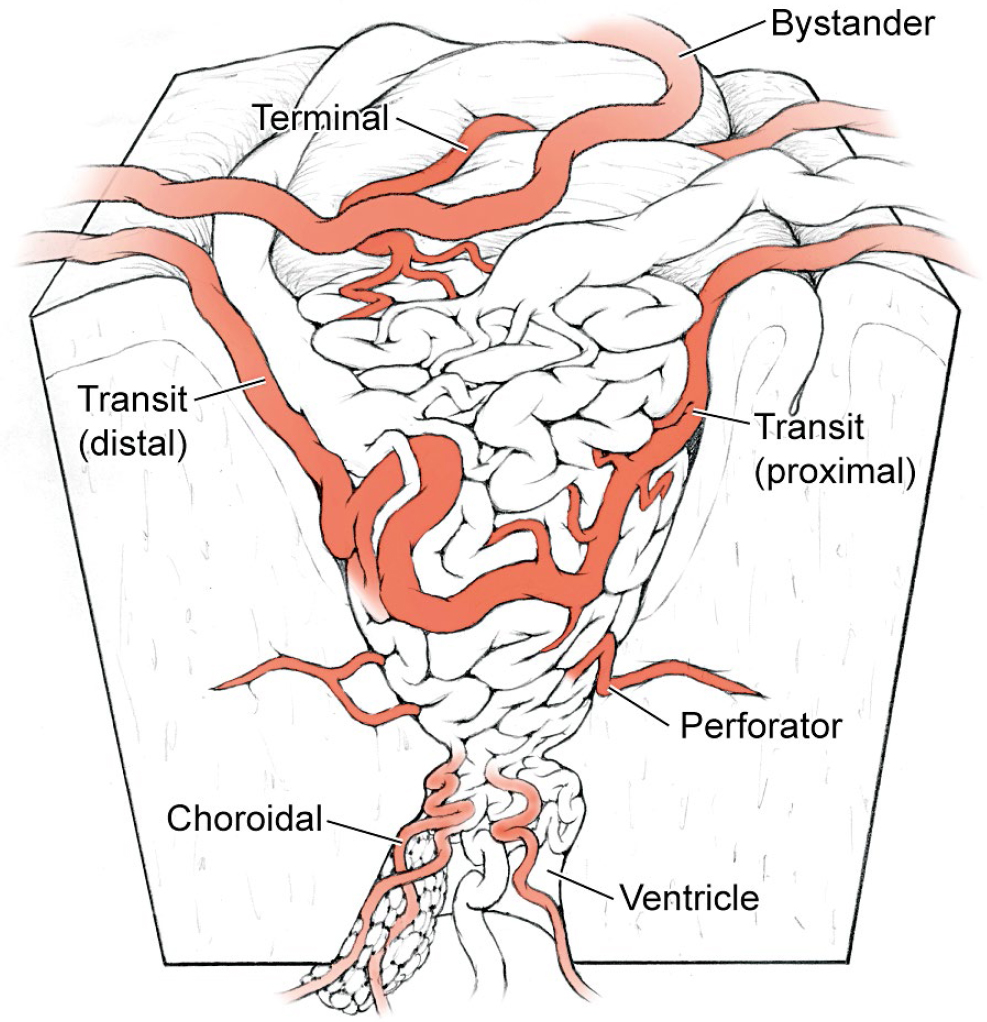
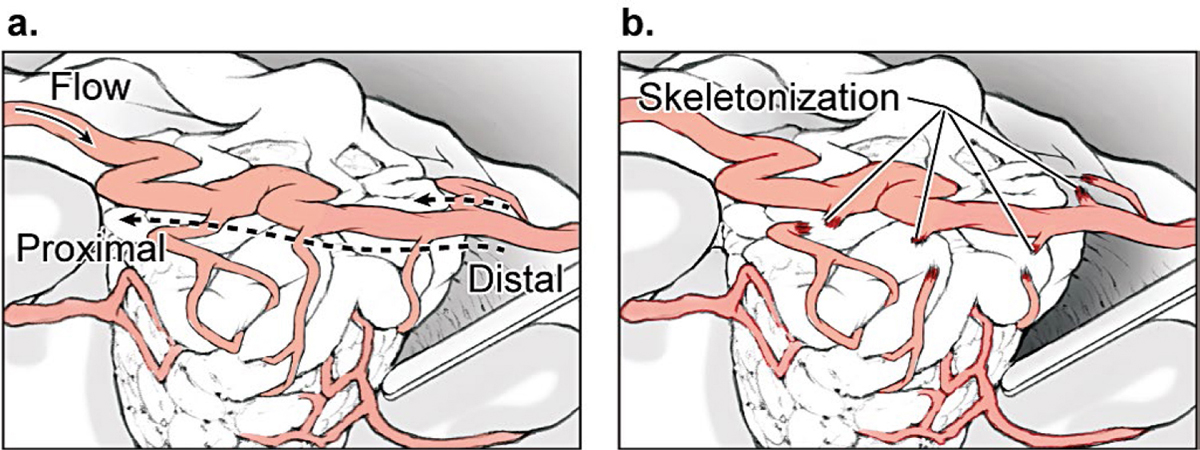
4 Feeding Arteries Arteries are occluded at their point of contact with the arteriovenous malformation (AVM). Occlusion more proximally sacrifices intervening normal branches and infarcts adjacent brain, whereas occlusion more distally can elicit bleeding from the nidus or preserve proximal feeding branches. The point of contact may be indistinct, and branches may be difficult to differentiate as normal or afferent. Large feeding arteries on the cortical surface are welcome targets because they can be eliminated early and easily. They are cauterized with bipolar forceps along a 3- to 7-mm segment. As a general rule, arteries coagulate best after flow arrest. The bipolars are walked down the artery in a proximal-to-distal direction, coagulating a downstream artery that has no flow. Walking the bipolars up the artery from distal to proximal is less effective because the upstream artery remains under pressure and can rupture with cautery. The application of electrical current across an artery shrinks wall tissues, tapers the artery, and ultimately obliterates its lumen. Arteries and arterioles have thick elastic and smooth muscle cell layers that coagulate and occlude easily. In contrast, tiny perforating arteries have thin walls with little elastic and smooth muscle cell layers to coagulate. Consequently, they resist bipolar cautery and are difficult to occlude. Flow arrest with a proximal microclip may transform an otherwise incoagulable perforator, but if not, the perforator is closed with a distal clip and divided. At the opposite extreme, large arteries with thick walls may also be difficult to occlude with bipolar cautery. Their caliber and high flow may be too much for coagulation alone and may require permanent aneurysm clips for closure (typically 5- or 7-mm straight clips). An artery that has been exposed to radiation during AVM radiosurgery undergoes morphological changes that enhance its coagulability. Radiation damages DNA in rapidly dividing endothelial cells, and as they attempt to regenerate, they are depleted over time. Intimal disintegration exposes smooth muscle cells and triggers a proliferative response in the medial layer. The progressive growth of the media thickens the arterial wall and constricts the lumen, which is the mechanism for radiosurgery’s occlusive action. Incompletely obliterated AVMs have irradiated arteries that are thickened with morphological changes resembling atherosclerosis. These are perhaps the most coagulable of all AVM feeding arteries. Embolized arteries are altered by intraluminal casts of embolic agents that vary greatly in their manageability. N-butyl cyanoacrylate (NBCA) glue forms a hard, translucent cast that is difficult to transect. Polyvinyl acetate (PVA) forms a much softer, particulate plug that can be cut, but can also crumble and reopen. Onyx forms a soft, rubbery cast that is also easily cut. Coils must be cut with heavy microscissors and may not be completely occlusive. Embolized arteries are divided to detach the AVM, and normal artery proximal to the embolic cast may need to be controlled in case flow persists around or through the embolic agent. Afferent arteries to and around AVMs can be categorized as terminal feeding arteries, transit arteries, perforating arteries, choroidal arteries, and bystander arteries (Fig. 4.1). The terminal feeding artery is dedicated to the AVM, terminates in the nidus, and has no normal territory to supply distally. It may have proximal branches that supply normal territory before it terminates. It travels in the subarachnoid space, is often large in caliber, and may have a single high-flow shunt or multiple peri- or intranidal branches. The terminal feeding artery is interrupted at the AVM margin with cautery or clips without concern for distal flow, but sometimes wraps around the nidus, transmitting normal branches before diving in. Terminal feeding arteries are quick victories. The transit artery is treacherous. It is not dedicated to the AVM and does not terminate in the nidus, and instead has proximal branches that supply normal territories, AVM branches that supply the nidus, and distal branches that supply normal territories. It is referred to as an artery “en passage” because it continues on a course past the AVM. Occlusion of the distal trunk results in a downstream infarction postoperatively. The caliber of a transit artery is smaller than a terminal feeder but larger than a bystander artery. Branches from a transit artery are typically twigs that make a contribution to the AVM but are not major shunts. The challenge of preserving the distal artery is accomplished by skeletonization: occluding the feeding twigs one by one to prune the parent trunk and preserve its distal flow (Fig. 4.2). These branches are easy to coagulate and rarely require clips. Feeding twigs can be difficult to differentiate from normal branches and the distal trunk, but skeletonizing in a distal-to-proximal direction solves this problem. The distal trunk is identified past the AVM and dissected retrograde toward the nidus. Branches that can be traced to the AVM margin are interrupted, and those that can be traced to adjacent cortex are spared. Feeders are more distended, tortuous, and dysmorphic than normal branches. Retrograde skeletonization leads to the proximal side of the AVM and enables the freed transit artery to mobilize off the AVM. The pericallosal arteries and insular segments of middle cerebral artery (MCA) are examples of transit arteries that are typically skeletonized with callosal and sylvian AVMs, respectively. Fig. 4.1 Types of AVM arteries: terminal feeding artery, transit artery, perforating artery, choroidal artery, and bystander artery. The terminal feeding artery ends in the nidus without normal territory distally and has large caliber and high flow. The transit or “en passage” artery has AVM branches as well as proximal and distal branches to normal territories. The perforating artery is a small-caliber feeding artery that traverses deep white matter and ends in the nidus with no normal territory to supply distally. The choroidal feeding artery is also small and terminal, but traverses the ventricle to supply an ependymal AVM surface. The bystander artery is a normal artery without AVM supply that courses alongside or adjacent to the nidus. The perforating artery is a small-caliber, terminal feeding artery that traverses deep white matter and ends in the nidus with no normal territory to supply distally. Its small caliber, deep location, and input on the ependymal surface make it different from the larger terminal artery in the subarachnoid spaces. Perforators are approximately 1 mm in diameter and include the medial lenticulostriates from the A1 anterior cerebral artery (ACA), the lateral lenticulostriates from the M1 MCA, the insular perforators from the M2 MCA, thalamoperforators from the posterior communicating artery (PCoA) and the P1 posterior cerebral artery (PCA), and brainstem perforators. Although perforating arteries are small, their blood flow is vigorous. Charlie Wilson used to call these “red devils”: they bleed briskly, resist cautery, course through eloquent white matter tracts, and are encountered on the deep plane of the AVM where they are hard to see. Their thin walls provide little substrate for electrocautery and they require liberal use of microclips, preferably before they start bleeding. When bleeding, they can be drawn into the sucker to dry the field and clipped proximally while suction holds the artery. An uncontrolled bleeding perforator is dangerous because it can retract into the white matter, damage brain tissue, and force pursuit deeper into vital tracts and nuclei. Red devils are more fearsome than large feeders. Fig. 4.2 (a) Skeletonization of a transit artery occludes feeding branches to the AVM and preserves flow in the distal parent artery. (b) The efferent trunk is protected by retrograde dissection in a distal-to-proximal direction.
 Arterial Occlusion Techniques
Arterial Occlusion Techniques
 Terminal Feeding Arteries
Terminal Feeding Arteries
 Transit Arteries
Transit Arteries
 Perforating Arteries
Perforating Arteries
 Choroidal Feeding Arteries
Choroidal Feeding Arteries
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree