Chapter 21 Guidance for the clinical use of electrophysical agents 2006
Reproduced with permission of the CSP, 14 Bedford Row, London WC1R 4ED
SECTION 1 INTRODUCTION
Chartered physiotherapists need to ensure that any interventions given will be clinically effective. Evidence of effectiveness can be drawn from research, expert opinion and patients’ and professionals’ experience. Recommendations in this document are based on a combination of the above, together with experience of the authors. A number of references are included in each section with additional suggested general literature in Section 9. Although these provide some key citations, they in no way provide a comprehensive review of the literature. The body of literature pertaining to electrophysical agents is both large and disparate. It is the responsibility of the user to keep up to date with this material.
1.1 DISCLAIMER
Although the information in this publication is relevant and accurate at the time of publication, readers and users of the material will need to take responsibility for identifying additional new information of relevance as it becomes available. It does not override the responsibility of the physiotherapist to make appropriate decisions for individual patients, in consultation with the patient and/or guardian or carer.
This guidance was arrived at after careful consideration of the available evidence and should be used in conjunction with the Chartered Society of Physiotherapy’s (CSP) Core Standards of Physiotherapy Practice (CSP 2005), Rules of Professional Conduct (CSP 2002), Core Standards, CSP guideline paper PA47, Medical Devices Agency guidance, 1998 (see Section 4) and relevant legislation, including the Consumer Protection Act and Health and Safety at Work Act (1974). See also websites, e.g.:
SECTION 2: SCOPE OF PRACTICE, CONSENT AND MONITORING
2.1 SCOPE OF PRACTICE
The professional responsibilities of physiotherapists in relation to the application of electrophysical agents should conform to the principles set out in the Rules of Professional Conduct (CSP 2002).
2.2 VALID CONSENT
Patients must have the capacity to give valid consent to treatment. They should be able to comprehend and retain material information, especially consequences, and be able to use and weigh information in decision making. Information given by the physiotherapist should enable the patient to make a balanced judgement, understand material or significant risks, and questions should be answered truthfully. The DH Consent website www.dh.gov.uk/consent has the full text of all DH Consent publications.
2.3 MONITORING PERFORMANCE
Unexpected effects reporting
In addition, it is the responsibility of the individual physiotherapist to report all unexpected effects of electrophysical treatments both in the appropriate way locally, and to the professional body. It is an important component of professional audits that all such unexpected effects are recorded and detailed accurately and fully. Included in this booklet is the current report form (Appendix 1) which should be completed and returned to the Clinical Effectiveness Unit of the CSP electronically via enquiries@csp.org.uk or to the postal address quoted in the event of a patient experiencing an unexpected response to treatment.
SECTION 3: RELATIONSHIPS WITH AND ADVICE TO THIRD PARTIES
3.1 LOAN/HIRE OF ELECTROPHYSICAL DEVICES FOR USE BY A PATIENT (e.g. home use of a TENS unit)
3.2 DELEGATION OF TASKS TO PHYSIOTHERAPY ASSISTANTS
3.8 ADVICE TO INDIVIDUALS NOT UNDER THE DIRECT CARE OF A RECOGNIZED PRACTITIONER
Physiotherapists approached in such cases should encourage the individual to seek assessment from a chartered physiotherapist or other suitably qualified practitioner, who can then advise on the suitability and appropriateness of the apparatus or agent purchased. Physiotherapists are encouraged not to provide informal advice to such patients, regardless of individual circumstances e.g. patient with chronic pain, unless a full assessment has been performed and the physiotherapist is familiar with the apparatus in question and its suitability.
SECTION 4: MAINTENANCE ISSUES
The standards for the maintenance of electrophysical equipment should be read in conjunction with Section 7, as they may be modified for specific electrophysical agents.
4.1 ELECTROPHYSICAL EQUIPMENT RECEIVES ROUTINE AND APPROPRIATE MAINTENANCE, WHICH INCLUDES QUALITY ASSURANCE OF FUNCTION AND SAFETY FEATURES
Criteria
The Medical Devices Agency, an Executive Agency of the Department of Health, has produced guidance on ‘Acceptance Testing’ and ‘The Management of Medical Equipment and Devices’ (www.medical-devices.gov.uk).
SECTION 5: COMPETENCE TO PRACTISE
Rule 1 of the Rules of Professional Conduct of the CSP states ‘Chartered physiotherapists shall only practise to the extent that they have established and maintained their ability to work safely and competently…’ (CSP 2002). Physiotherapists should therefore confine themselves to the use of agents in which they have undertaken relevant pre- and/or post-qualifying programmes. Learning from manufacturer’s literature alone is not adequate. Physiotherapists have a responsibility for updating themselves, as new knowledge becomes available.
Continuing professional development is described by the CSP as ‘the lifelong learning that professionals need to undertake throughout their career in order to maintain, enhance and broaden professional competence’. As in other areas of the profession, competence to practise is maintained through the incorporation of information obtained from a variety of sources, including:
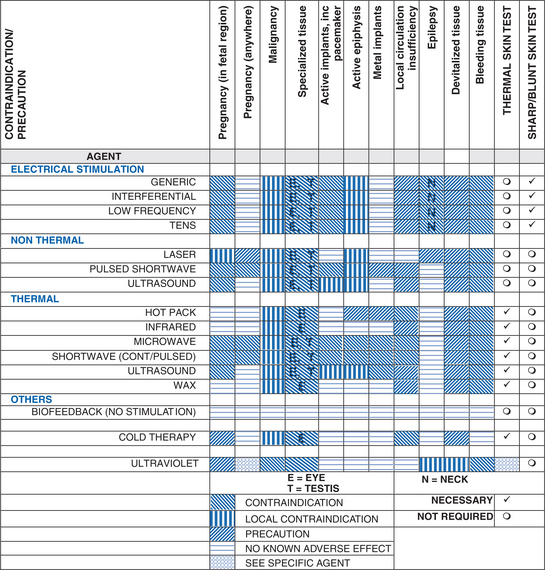
SECTION 6: CONTRAINDICATIONS, PRECAUTIONS, SAFETY AND APPLICATION ISSUES
The following guidelines apply to all the equipment referred to in Section 7. Additional safety measures are also specified in Section 7 for specific agents, and these should also be adhered to.
6.1 CONTRAINDICATIONS FOR ALL AGENTS
| Reference | |
|---|---|
| • Those who are unable to comprehend instructions, or who are unable to co-operate | CSP Core Standards (2005) |
| • The application of electrophysical agents over the abdomen, lower back or pelvis is normally contraindicated during the first 35 weeks of pregnancy. Refer to specific information for each agent | Expert opinion |
| • In the area of a tumour where there is active or suspected malignancy, except for palliative care | 001*/002*/003* 004*/005*/006* 007*/008*/009* |
| • Areas of recent bleeding tissue or haemorrhage | Expert opinion |
| • Active tuberculosis in treatment area | Expert opinion |
*001 Auda SP, Hall G, Elias J et al (1979) Selective tumor heating and growth retardation by shortwave radiofrequency. Surg Forum 30: 154-156.
6.2 PRECAUTIONS FOR ALL AGENTS
Treatment should not normally be carried out:
| Reference | |
|---|---|
| • Over the anterior aspect of the neck | Expert opinion |
| • Where there is significant impairment in the circulation/sensory loss of the area to be treated | Expert opinion |
| • Where there is devitalized tissue, e.g. after recent radiotherapy | Expert opinion |
| • Where there are local acute skin conditions, e.g. eczema, dermatitis | Expert opinion |
6.3 HEALTH AND SAFETY CONSIDERATIONS
| Reference | |
|---|---|
| • Treatment is carried out in compliance with local regulations | Norm |
| • The surrounding environment is safe for treatment | Expert opinion |
| • There is a warning sign relating to patients with internal stimulators where high-frequency equipment is used | Expert opinion |
6.5 APPLICATION FOR ALL AGENTS
| Reference | |
|---|---|
| Measures should be taken to ensure that the electrophysical equipment is applied in a manner that is conducive to safe usage | Expert opinion |
| The user manual for each piece of equipment is read and understood before its use | Expert opinion |
| Equipment and accessories are checked as appropriate prior to application | Core standard 18 |
| Relevant technical safety checks are carried out for the specific apparatus | Core standard 18 |
| Equipment is kept clean | Expert opinion |
| Where appropriate, the intensity indicator is set at zero prior to switching on or off | Expert opinion |
| The equipment is switched on/off in the correct sequence | Expert opinion |
| Where appropriate, intensity dials are turned up or down gradually | Expert opinion |
| The patient should be positioned comfortably with adequate support to remain in the given position for the duration of the treatment | Expert opinion |
| Preparation of patient | |
| • an explanation of the planned treatment is given to enable valid consent | 010*/011* |
| • the sensation of the planned treatment to be experienced is explained | 010*/011* |
| • the patient is warned of any effects that should be reported | 010*/011* |
| Examination and testing | |
| • this refers to specific examination of the part of the body to be treated for possible hazards, contraindications and precautions, plus any appropriate tests. A check should be made to ascertain whether the patient might suffer an allergic reaction to any substance being applied to the skin | 010*/011* |
| Drug information | |
| • information must be obtained from the patient concerning medication that may interfere with or mask the effects of the electrophysical agent. | Expert opinion |
| Assembly of apparatus | |
| • visual checks are made of electrodes, leads, cables, plugs, power outlets, switches, controls, dials and indicator lights | 010*/011* |
| Preparation and testing of apparatus | |
| • the operator(s) should minimize their own exposure to the agent | 010*/011* |
| Preparation of the part to be treated | |
| • this involves any preparatory procedure, e.g. washing the area | 010*/011* |
| Setting up | |
| • the apparatus should be set up to ensure optimum therapeutic effect and safety | 010*/011* |
| • where possible the same piece of equipment is used at each visit. | Expert opinion |
| • the functional output of the equipment is tested on the day of use | Expert opinion |
| • local policies relating to infection control are adhered to, and specialist advice is sought where appropriate | Expert opinion |
| Application | |
| • the patient should be monitored appropriately to ensure that treatment is progressing satisfactorily and without unexpected effect | 010*/011* |
| Treatment | |
| • if pain, discomfort or unexpected sensations are experienced by the patient treatment intensity should be modified | Expert opinion |
| • at the termination of treatment the part treated should be examined, and the general condition of the patient evaluated | 010*/011* |
| For those devices that allow a hands-free application: | |
| • a member of staff remains within calling distance during treatment | Expert opinion |
| • the patient is provided with a means of calling for assistance | Expert opinion |
| Recording | |
| • an accurate record of assessment findings, machine settings, all treatment parameters and effects must be made. This is required to enable accurate treatment replication and as a legal requirement. | 010*/011* Core standard 14 Core standard 18 |
*010 Kitchen S (ed) (2002) Electrotherapy: Evidence-based Practice. London, Churchill Livingstone.
*011 Low J, Reed A (2000) Electrotherapy Explained – Principles and Practice, 3rd edn. Oxford, Butterworth-Heinemann: 27-28.
Supplementary reading: all agents – application
Chartered Society of Physiotherapy (CSP) Safety of Electrotherapy Equipment working group. Guidelines for the safe use of ultrasound therapy equipment. Physiotherapy. 1990;76(11):683-684.
Chartered Society of Physiotherapy (CSP) Safety of Electrotherapy Equipment working group. Guidelines for the safe use of microwave therapy equipment. Physiotherapy. 1991;77(9):653-654.
SECTION 7 AGENTS
The information in the following sections should be read in conjunction with the generic information in Section 6.
7.1 BIOFEEDBACK
7.1.3 Precautions
| Reference | |
|---|---|
| • Ensure that the skeletal and soft tissues are capable of withstanding the enhanced forces that may be generated by the facilitated muscle. | Expert opinion |
7.2 COLD THERAPY
7.2.2 Contraindications
| Reference | |
|---|---|
| • Acute febrile illness | Expert opinion |
| • Vasospasm (e.g. Raynaud’s disease/development of white/blue fingers with cold) | Expert opinion |
| • Cryoglobulinaemia | Expert opinion |
| • Cold urticaria/allergy | Expert opinion |
7.2.3 Precautions
| Reference | |
|---|---|
| • Appropriate precautions should be taken in the presence of open wounds, infected tissue and skin lesions | Expert opinion |
| • Very large areas (e.g. bilateral lower limbs) should never be subjected to very low temperatures | Expert opinion |