CHAPTER 44 Foramen Magnum Meningiomas
INTRODUCTION
Among the meningiomas of the posterior fossa, foramen magnum (FM) meningiomas deserve special consideration because of their characteristics in symptomatology, intriguing surgical anatomy, unique operative requirements, and outcome. When all meningiomas are considered, FM meningiomas have the worst outcome in terms surgical results and operative morbidity.1
DEFINITIONS
FM meningiomas arise at the dura of the craniocervical junction. There was much variability in the older literature regarding the definition of FM tumors. Some authors considered any tumor that was inserted at or passed through the FM as FM tumors.2 Others defined them as craniospinal tumors with anterior extension or spinocranial tumors with posterior extensions.3,4 In the older literature this area was extended down to C3 and even to C4.5–8 The anatomic definition of the FM region by George and colleagues9 gained general acceptance in recent publications. The definition of a FM meningioma is that of a meningioma having its attachment in the FM region. This region is clearly defined: the upper boundaries of the FM region are the lower third of the clivus and the occipital bone while the inferior margins are composed of the body, lamina, and the transverse process of the axis.10 The lateral boundaries are composed of jugular tubercles and the upper aspect of C2 laminas.10 Meningiomas that arise elsewhere but that have an extension into the FM are not included among FM meningiomas. Meningiomas that extend into the FM but with their main origins outside the FM area such as those of the hypoglossal canal, jugular foramen, middle and upper clivus, and cerebellopontine angle are considered as separate entities.
On the axial plane, the FM is also divided into ventral, lateral, and posterior compartments. Ventral and posterior compartments are separated by the coronal plane that spans the first dentate ligament and IX–XIIth cranial nerves.1 FM meningiomas located ventral to the neuraxis pose complex surgical problems to the surgeon due to their close proximity to the brain stem, lower cranial nerves, and the vertebral artery (VA), which increases the surgical risks and the narrow surgical corridor. Controversy still exists regarding the optimal surgical approach for ventral FM meningiomas.
SURGICAL ANATOMY
The Vertebral Artery
For an anatomic description, the VA is divided into four segments.11 The first, “pretransverse” (ostial, proximal) segment extends from its origin to the transverse process of the C6 vertebra. The second “transverse” segment runs inside the transverse processes of C6 through the axis.12 Of special interest for surgery around the FM are the third and fourth segments. The V3 segment is also called the “suboccipital segment” and extends from the transverse process of the axis to the dural penetration of the VA. This segment has three portions: vertical, horizontal, and oblique. The vertical portion lies between the transverse process of axis and atlas. The horizontal portion lies in the groove of the posterior arch of the atlas. In this groove, the atlantooccipital membrane covers the VA. The posterior atlantooccipital membrane stretches between the inferior border and the posterior surface of the occipital one and the posterior arch of the atlas. Ossification or calcification of the occipitoatlantal membrane with complete encasement of the VA in the posterior arch of atlas has been reported in 7.8% to 28% of cases.13,14 The oblique portion leaves this groove and climbs up to the dural penetration. The VA penetrates the atlantooccipital dura on its lateral aspect and becomes the V4 segment, which is also called the “intracranial segment.”11,14 The two V4 segments join at the midline to form the basilar artery (BA).
The VA has four vascular loops. At the transverse foramen of the axis, the artery turns posterolaterally, forming the inferior medial loop. Just next to this loop, the artery turns in the anterosuperior direction before entering the transverse foramen of the atlas, forming the inferior lateral loop. The superior lateral loop is formed where the vertical segment of V3 turns into the horizontal segment. Immediately before penetrating the dura, the VA forms the superior medial loop. This last loop turns around the condyle of the atlas and is attached tightly to the atlantooccipital articulation by the retroglenoid ligament. Therefore, the VA is fixed at its two extremes of the V3 segment: at the transverse foramen of atlas and at the dural penetration. At the same time, the V3 segment is the most mobile part of the VA and rotational movements change anatomic relationships.10 In the neutral position, the vertical and horizontal segments are perpendicular. After head rotation (and also after positioning for the anterolateral approach), the transverse processes of the atlas and axis are pushed away from each other and both segments become stretched and run parallel, separated by the posterior arch of the atlas.15
The V3 segment is cushioned by a venous plexus in its entire length. Parkinson16 first drew attention to its similarity to the cavernous sinus and Arnautovich17 nicknamed it the “suboccipital cavernous sinus.” At the transverse process of atlas, the artery, the surrounding venous cushion, and the periarterial autonomic neural plexus are covered by a periosteal ring. At the dural penetration, the periosteal sheath that covers the V3 segment in its horizontal portion invaginates into the dura and creates a second fibrous ring. At this level, the periosteal sheath is in continuum with both the outer layer of the dura and the adventitia of the VA. As discussed later, attempts at resection of meningiomas that invade this dural portion carry the risk of rupturing the VA.
The posterior circulation may be subject to variations and anomalies.11,18 In 40% of individuals there is an asymmetry of the VA size. In such instances the VA is most commonly larger on the left side.11 In 20% of the cases, the posterior inferior cerebellar artery (PICA) arises extracranially from the V3 or even V2 segments.19 Therefore the PICA must be identified and protected during surgery. Another rare finding is the proatlantal artery, which is a persistent embryonic anastomosis between the VA and the internal carotid artery (ICA). A persistent proatlantal artery is commonly associated with an atretic VA and an extracranial origin of the PICA.
Arterial Branches of the Vertebral Artery
There are several small branches of the V3 segment. The vertical portion has two constant branches.17 The first of these is the muscular branch which originates ventral to the anterior ramus of C2 nerve. It communicates with branches of the ascending pharyngeal artery. The second and also the largest branch of the V3 segment is the radiculomuscular branch originates below the transverse foramen of atlas and divides into radiculomedullar and muscular branches. Both of these branches are coagulated and divided during VA transposition. The horizontal portion also has 2 constant branches.17 The small muscular branch originates just superior to the transverse process of atlas and corresponds to the radicular artery of C2. It supplies surrounding muscles. This small muscular branch usually communicates with the occipital artery. The posterior meningeal artery originates from the posterosuperior surface of the superior medial loop and supplies the posterior fossa dura, posterior tentorium, and the squamous portion of the temporal bone. Anastomoses are reported with (1) branches from occipital artery, (2) the V2 portion of the VA through the anterior meningeal artery, (3) the ascending pharyngeal artery, (4) and the dorsal meningeal artery which is a branch of the meningohypophyseal trunk that arises form the ICA within the cavernous sinus. The posterior spinal artery may also arise from the horizontal portion, and in these cases, it enters the dura where the C1 root exits the spinal canal. The posterior spinal artery most commonly originates intradurally from the V4 segment or the PICA and is located medial to the most rostral attachment of the dentate ligament. Another branch that enters the posterior condylar canal is also reported.17Arterial branches of the V4 are PICA, the anterior spinal artery, and the anterior and posterior meningeal arteries. The major branch of the V4 segment is the PICA, which may also originate extradurally.19
HISTORY
The first case description of an ventral FM meningioma was provided in 1872 by Halopeau at autopsy.20 This was followed in 1922 by Frazier and Spiller’s21 first report of surgical intervention. Several other attempts were all complicated by intraoperative respiratory depression.22,23 Elsberg and Strauss4 performed the first successful surgical removal in 1925 with complete resolution of the patient’s symptoms. Surgical results on small cohorts were reported in the following decades, but meningiomas of the region were either clustered together with other tumors of the craniovertebral junction or with other meningiomas such as posterior fossa or clivus. In his review of the existing literature from 1924 to 1976, Yasargil documented 114 reported cases of FM meningiomas.24 The unique characteristics and challenges of FM meningiomas that separated them from other posterior fossa meningiomas were discovered early. The largest series and the most comprehensive analysis of FM meningiomas was provided by George and colleagues,25 and his work established FM meningiomas as a separate disease entity. In his analysis of the former literature, George identified three periods in the neurosurgical literature, mainly of the first attempts at surgery (1925–1950), then a period concerned with attempts to improve diagnosis (1950–1980) and the last one of technical improvements (1980–present).9 Microsurgery was a major step in decreasing mortality and morbidity but with their popularization and maturation in the last two decades, skull-base approaches and their results have dominated the literature.26 Skull-base techniques have significantly expanded the neurosurgical armamentarium by increasing our surgical capabilities and decreasing morbidity.27–31
INCIDENCE
FM meningiomas are rare and make up 0.3% to 3.2% of all meningiomas, 4% to 6.5% of posterior fossa meningiomas, and 8.6% of all spinal meningiomas.9,32,33 FM is the second most common site for posterior fossa meningiomas.24
Among lesions located at the FM, benign extramedullary tumors comprise one third.9,33–36 Among these lesions meningiomas are the most common tumor type and constitute about 70% of all benign tumors arising in this region.33,37 This is almost three times more frequent than the next most common tumor, neurinomas.1 Most meningiomas are intradural. However, there may be extradural extensions.9,33–36 Strictly extradural tumors are exceedingly rare. FM meningiomas are most commonly found in the fourth to sixth decades of life. FM meningiomas can also be observed in young children. Pediatric FM meningiomas are usually associated with neurofibromatosis.32,38,39 FM meningiomas in children are reported to have a poorer prognosis compared with those in adults, as the tumors tend to grow more rapidly and to a larger size, undergo malignant changes, and have a greater rate of recurrence.40 The female predominance observed in meningiomas in general also holds true for FM meningiomas. The female-to-male ratio is 2–4:1.9 FM meningiomas may present in patients with multiple meningiomas, and this may be a component of the neurofibromatosis type 2 (NF2) complex.
CLINICAL MANIFESTATIONS AND NATURAL COURSE OF THE DISEASE
The clinical manifestations of FM meningiomas are atypical and unpredictable. There are no symptoms or signs that are exclusively diagnostic of FM meningiomas. Before the common use of magnetic resonance imaging (MRI), these lesions were commonly discovered very late in their course, due to their slow growth inside a wide subarachnoid space. A significant delay is reported between the onset of the initial symptom and neurologic findings.35,41,42 In the older literature this period ranged from 20 days to 6.5 years.35,41,42 Newer studies report an average of 30 months between the onset of symptoms and the diagnosis.9 A relapsing and remitting clinical picture has been reported in a significant proportion of the patients and it is common for them to come to clinical attention after minor head–neck trauma.43 Before the frequent use of MRI, the most common first diagnoses in patients with FM tumors were cervical spondylotic myelopathy (12.5%–21%), multiple sclerosis (17.5%–18.8%), syringomyelia (15.8%), intramedullary tumor (14%), cervical disc herniation (12.5%), Arnold Chiari malformation (5.3%), carpal tunnel syndrome (5.3%), subacute combined degeneration (3.5%), amyotrophic lateral sclerosis (3.5%), normal pressure hydrocephalus (1.8%), and brachial plexus injury (1.8%).33,39 The increased availability of advanced neuroimaging has decreased this delay.
The most common symptom is occipital or posterior neck pain, and this presents as the initial symptom in 65.7% to 80% of patients.1,9,35,41,42,44 The pain usually presents in the morning and becomes worse with neck movement (especially extension), maneuvers that increase intracranial pressure or coughing.1,9 Hyperestesia in the C2 dermatome may also be seen with posterior cervical pain. Paresthesia in distal extremities is a late symptom. In advanced cases, spastic weakness in the extremities may be seen. This usually becomes manifest as loss of fine motor skills in the fingers. The presentation of a “rotating palsy,” that of a paresis that starts in one upper extremity and then progress to the ipsilateral lower extremity, then to contralateral lower extremity and finally to the contralateral upper extremity, strongly suggests a FM lesion.
Neurologic examination is commonly positive for ataxia, motor and sensory deficits, cerebellar signs, and lower cranial nerve palsies: Motor weakness is detected as a spastic paresis in the extremities with wasting of intrinsic hand muscles. A spastic gate may be found in more than half of the patients as well as hyperreflexia and a positive Babinski’s sign. Dissociated sensory loss and a Brown Sequard syndrome may be encountered. Lower cranial nerve deficits are uncommon in patients with FM meningiomas except for CN XI palsies, which are reported in up to 44% of patients.42 Findings include dysarthria, dysphagia, atrophy of the sternocleidomastoid or trapezius muscles, hoarseness, and Horner’s syndrome. Respiratory difficulties are a late finding.
PATHOLOGY
Most common histologic types are meningothelial, followed by psammomatous and fibrous meningiomas.24,45–47 The incidence of the meningotheliomatous histology ranges from 22% to 72%, while transitional type is seen in 17% to 28% and the psammomatous type in 11% to 22% of cases.1,33,42,44,48 No association has been reported between the histologic subtype and extent of resection for FM meningiomas.
DIAGNOSTIC STUDIES
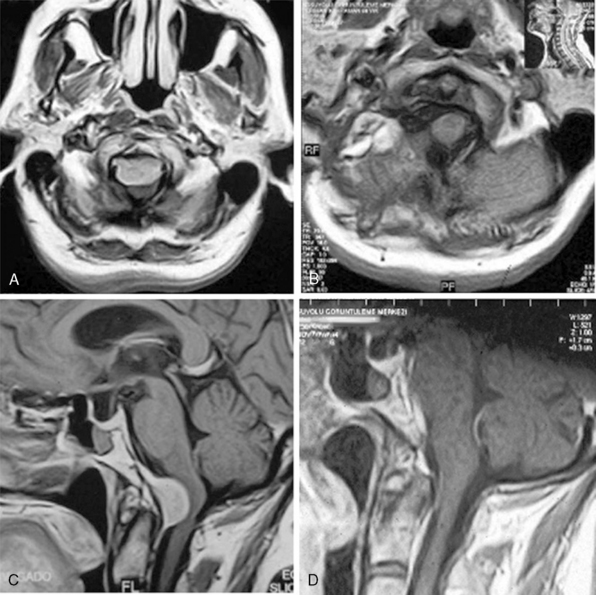
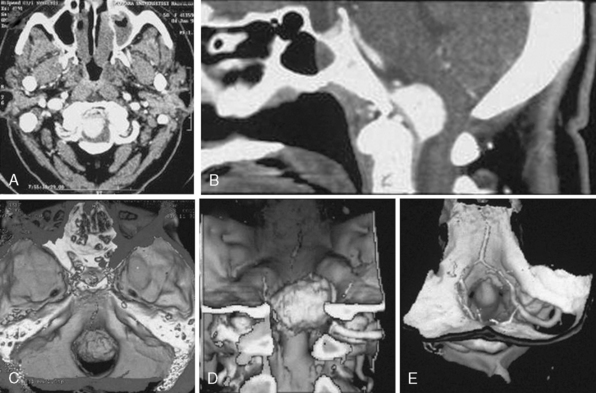
MRI and computed tomography (CT) constitute the basic workup in patients with FM meningiomas. Angiography is seldom required. The information provided by the MRI and CT is useful for the differential diagnosis (from other lesions in the FM) as well as surgical planning (such as the localization of the tumor both on axial and sagittal planes, extent of neuraxis compression and the nature of its displacement, presence of extradural extension, possible encasement of the VA, and presence of other coexistent pathologies such as hydrocephalus) (Figs. 44-1 and 44-2). Among benign extramedullary tumors, meningiomas are the most common pathology. Only a small proportion of meningiomas have an extradural extension. In cases that have an extradural extension, other common pathologies such as metastases and chordomas must be excluded.38,49 The differential diagnosis of intradural mass lesions at the FM includes intra-axial tumors (astrocytomas, ependymomas, subependymomas), extra-axial tumors (meningiomas, schwannomas, glomus jugulare tumors, teratomas, lipomas, hemangioblastomas), non-neoplastic cysts (epidermoid inclusion cysts, neurenteric cysts, arachnoid cysts, dermoid tumors), vascular lesions (cavernomas, giant thrombosed VA aneurysms), tonsillar herniation, and syringomyelia. The differential diagnosis of extradural mass lesions at the FM includes metastases, chordomas, osseous–cartilaginous tumors, sarcomas (including chondrosarcomas), congenital malformations of the craniovertebral (CV) junction, infections, proliferative arthropathies with associated degenerative disease (e.g., Rheumatoid pannus), and synovial cysts. Like meningiomas in other localizations, FM meningiomas appear iso- to slightly hypointense relative to brain parenchyma on T1-weighted images and enhance intensely after intravenous contrast injection. On T2-weighted images meningiomas appear as iso- to hyperintense and the high intensity is correlated with soft consistency of the tumor. Softer tumors are “suckable” and easier to remove surgically. Magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) provide information on the status of arterial and venous structures. CT provides extra information on the presence of hyperostosis or calcifications. The tumor appears iso- to hyperintense and enhances intensely after contrast injection. Bone involvement is also clearly delineated in tumors that have an extradural component. Calcifications, which are seen in 10% of meningiomas, are also easily demonstrated on CT. Anatomic information on the condyles, jugular tubercle and the jugular bulb are also very valuable for preoperative planning, and three-dimensional reconstructions are very helpful in this regard. Plain radiographic studies are usually nondiagnostic in FM meningiomas; however, they may demonstrate bone destruction or hyperostosis in extradural tumors. Angiography is not routinely included in the preoperative workup of FM meningiomas. Vascular supply of FM meningiomas comes from both the vertebral and external carotid (and occasionally internal carotid) arteries, and a complete six- vessel angiogram is required for a complete study. Angiography is superior to MRI in evaluating vascular encasement.38
SURGICAL TREATMENT
Surgical Classification Schemes
Several authors devised classification schemes for FM meningiomas to aid in defining the surgical approach. Cushing and Eisenhard3 classified meningiomas at the craniovertebral junction into “craniospinal meningiomas” that originated from the basal groove at the lower third of the clivus, anterior to the medulla and projected caudally and “spinocranial meningiomas” that originated in the upper cervical area, posterior to the spinal cord and projected cranially into the cerebellomedullary cistern. This classification system is still used by some authors.1
Others described classification schemes according to the zone of dural attachment of the tumor on the axial plane. The boundaries of anterior, lateral, and posterior compartments on the axial plane were previously described. The meningioma may be located ventral (dural attachment on both sides of the anterior midline), ventrolateral (ventral to the dentate ligament), posterolateral (dorsal to the dentate ligament), or posterior (on both sides of the posterior midline). However, the surgical approaches of the posterior and posterolateral cases are the same, and most authors group posterolateral and posterior cases into one category. In their latest classification George and Lot9 differentiated among ventral, lateral, and posterior FM meningiomas.
A ventral FM meningioma is one of the most difficult types of meningiomas to treat. According to different studies, ventral and ventrolateral FM meningiomas comprise 68% to 98% of all cases.42 Most studies have grouped ventral and lateral meningiomas together. In different studies that have differentially analyzed ventral and lateral meningiomas have indicated that 4.7-85.7% of these are in fact anterior FM meningiomas.30,32,35,39,41,42,44,50,51 This wide variability may be due to a selection bias, and it is thought that the incidence of strictly anterior meningiomas is toward the lower end of this range. Ventral FM meningiomas are hidden deep in the craniocervical junction, surrounded by several important neurovascular structures. In strict ventral cases, the lower cranial nerves and the brain stem are usually displaced posteriorly and these vital structures cover the surgical path in a posterior approach. Posterolateral approaches were described, and preoperative classification aids in choosing the optimal approach.
The relation of the tumor to the VA is another factor that influences surgery. In tumors that are located below the VA, the lower cranial nerves are pushed posteriorly and superiorly, out of the surgical path, and no special effort is required for their protection. However, in meningiomas that grow above the VA, the displacement of the lower cranial nerves cannot be anticipated, and all of them need to be identified and protected during surgery. In rare cases, FM meningiomas can also infiltrate the dura at the level of dural penetration of the VA. As indicated earlier, the adventitia of the VA may be in continuum with the dura and resection of the tumor may result in vertebral artery tear. Bruneau and George10 have included all three of these parameters, namely the zone of dural insertion, compartment of development, and the relation of the tumor to the VA into their latest classification.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree