CHAPTER 50 Fractionated Radiation for Meningiomas
PRINCIPLES OF EXTERNAL BEAM RADIOTHERAPY
Molecular Mechanism and Cellular Endpoints
Radiation principally acts by ionizing molecules in irradiated tissues to cause DNA damage. Although radiation damage can be directly ionizing, in clinical therapy damage is most commonly caused indirectly, via the induction of highly reactive free radicals. The principal radiation-induced DNA damage is in the form of single- and double-strand breaks, the latter of which are generally considered the lethal event, leading to cell death.1 The majority of radiation-induced DNA lesions, particularly single-strand breaks, are effectively repaired via a number of complex repair mechanisms.2,3 It is assumed that DNA damage is responsible for radiation-induced inhibition of tumor growth in benign as well as in malignant tumors, and although likely, in benign tumors it remains unproven.
The end result of radiation damage after therapeutic radiation of malignant tumors is cell death, which is measured experimentally as clonogenic cell survival. Repeated small doses of radiation are less damaging to cells than an equivalent total dose given in a single fraction. Different dose fractionation schemes can be compared via a mathematical model (the linear quadratic [LQ] model), which compares different dose fractionation schemes through the concept of a biologically equivalent dose. The LQ model, which takes into account the total dose and the dose per fraction (and hence the number of doses/fractions), contains constants α and β, and it is the α/β ratio that distinguishes the responses of different tissues to radiation.1,4
Acute reacting tissues such as epithelium have a high α/β ratio generally in the range of 8 to 10 Gy (ranging from 7 to 20 Gy), and late-reacting tissues, exemplified by the normal central nervous system (CNS), have a low α/β ratio, usually 1 to 3 Gy (ranging from 0.5 to 6 Gy). The slow growth and presumed limited dividing capacity of benign tumors such as meningiomas has led to the assumption they have a low α/β ratio, but this remains unproven. Late-responding tissues are more spared by fractionation than early-responding tissues, and this is the principle of fractionated radiotherapy, particularly as applied to the CNS which has an α/β ratio of 1 to 2 Gy where fractionation preferentially spares normal brain compared to a high α/β ratio tumor.1
Technical Aspects of Radiotherapy
MLC leaves can also be used to alter the intensity of radiation, and this is described as intensity-modulated radiotherapy (IMRT). IMRT allows for better conformation of radiation to complex shape targets, particularly with concave regions containing critical normal tissue. Planning studies of complex meningiomas have demonstrated that IMRT may improve sparing of normal brain tissue in selected5,6 but not all cases.7
Proton radiation has the appeal of more localized deposition of energy than can be achieved with photons (X-rays), therefore offering the potential for better sparing of normal tissue particularly beyond the principal target. The rationale for the use of protons in meningiomas is based solely on the theoretical benefit of more localized conformal delivery of radiation shown in some cases.8,9
CLINICAL PRACTICE AND OUTCOME OF CONVENTIONAL FRACTIONATED RADIOTHERAPY IN BENIGN MENINGIOMAS
Tumor Control
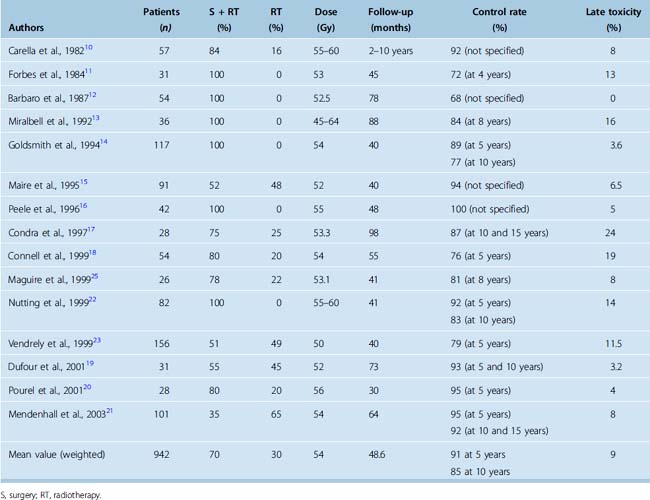
Retrospective studies of conventional fractionated radiotherapy in patients with residual and recurrent meningiomas have demonstrated apparent benefit in local tumor control after radiotherapy10–21 (Table 50-1). However, none of the studies are randomized or prospectively controlled and none have so far reported convincing survival gain with the early addition of irradiation. Nevertheless the reported progression-free survival (PFS) after radiotherapy of benign meningioma is in the region of 90% at 5 years and 80% to 90% at 10 years14,17,19,21,22 (see Table 50-1).
There is limited information on the appropriate dose of irradiation. The reported tumor control is similar for doses ranging from 50 to 60 Gy at less than or equal to 2 Gy per fraction. Doses less than 50 Gy were reported to be associated with worse disease control,13,21,23 although this is based on retrospective studies. The recommended dose for benign meningiomas is generally 50 to 60 Gy (usually 54–56 Gy) at less than 2 Gy per fraction. Our practice is to limit the dose to 50 Gy for meningiomas involving the optic pathways.
Tumor site and size have been reported as predictors of tumor control.14,15 In skull-base tumors, the generally larger sphenoid ridge tumors fare worse than small cavernous sinus lesions.22 There is little evidence that timing of radiotherapy is important, as local control and survival rates are similar whether radiotherapy is given as part of primary treatment or at the time of recurrence.14,21,22 Age and gender have little influence on long-term disease control.
Neurologic Function
An important endpoint of treatment of benign meningiomas is the preservation or improvement of neurologic function. More than 70% of patients with skull-base meningiomas have a neurologic deficit that may be a consequence of tumor growth or previous surgery. An improvement or more often stabilization of neurologic deficits has been reported in up to two thirds of patients after conventional radiotherapy,15,19,23 although this is generally in the absence of objective prospective recording of function. Our experience suggests that the majority of patients have stabilization of neurologic deficit for the duration of disease control.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree