Fig. 1
Effect of fucoidan on brain water content (BWC) 24 h after ICH. There was a significant increase in brain water content in the ipsilateral basal ganglia after ICH. The fucoidan-treated group displayed a similar increase in BWC; treatment did not reverse the effects of ICH. Data are represented as mean ± SEM (n = 7/group; * = p < 0.05 vs. sham). Ipsi-BG ipsilateral basal ganglia, Ipsi-CX ipsilateral cortex, Contra-BG contralateral basal ganglia, Contra-CX contralateral cortex, CB cerebellum
Fucoidan Failed to Improve Neurological Outcomes at 24 h
Significant sensorimotor deficits were observed at 24 h after ICH in all behavior tests (p < 0.05 vs. sham) (Fig. 2). Treatment with fucoidan at doses of 25, 75, and 100 mg/kg was not able to improve Garcia neuroscores (p < 0.05 vs. sham) (Fig. 2a). Fucoidan was also not able to improve neurofunctional outcome in the corner turn and forelimb placement tests at 24 h with all three doses (Fig. 2b, c).
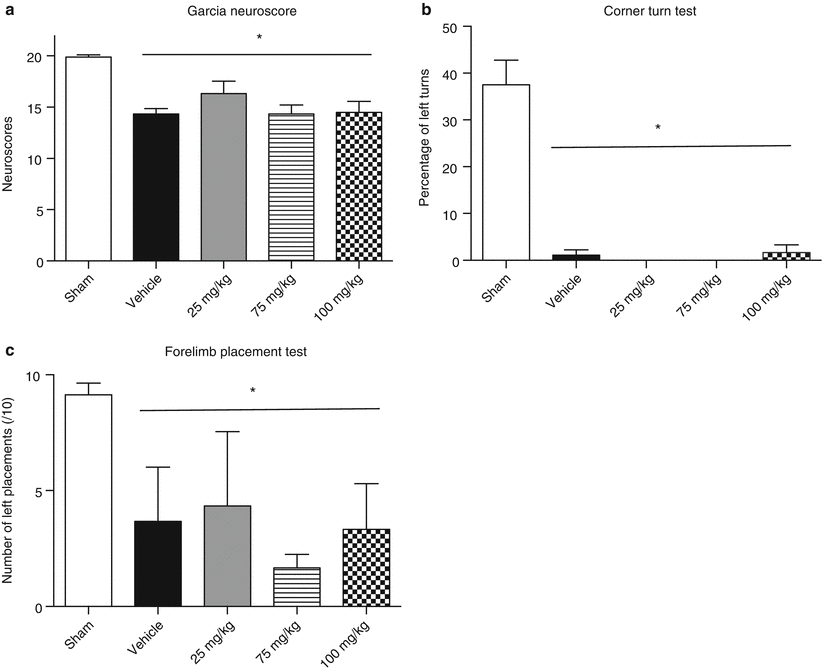

Fig. 2
Effect of treating with fucoidan on neurological function 24 h after ICH. ICH resulted in significant loss of neurological function, as assessed by Garcia neuroscore (a), corner turn test (b), and forelimb placement test (c). Mice were treated with 25, 75, or 100 mg/kg fucoidan, which failed to improve the sensorimotor deficits induced by ICH. Data are represented as mean ± SEM (n = 5–9/group; * = p < 0.05 vs. sham)
Treatment with Fucoidan Did Not Affect Hemoglobin Content 24 h after ICH
The hemoglobin content in the ipsilateral hemisphere of ICH mice was significantly increased compared with sham (p < 0.05). Fucoidan at 100 mg/kg did not alter the hemoglobin content, in comparison to vehicle-treated animals (p < 0.05 vs. sham) (Fig. 3).
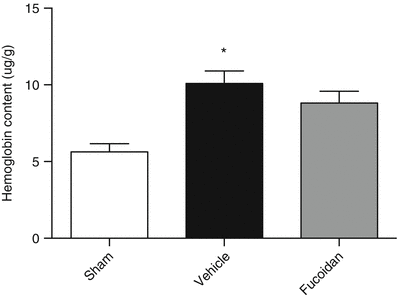

Fig. 3
Effect of fucoidan treatment on hemoglobin content 24 h after ICH. ICH significantly increased hemoglobin content in comparison with sham. Treatment with fucoidan was not able to attenuate this increase. Data are represented as mean ± SEM (n = 5–9/group; * = p < 0.05 vs. sham)
Discussion
In the present study, we examined the effects of fucoidan, a polysaccharide isolated from the brown seaweed Fucus vesiculosus, on outcomes following experimental ICH in mice. We found that fucoidan failed to improve the worsened neurological outcomes induced by ICH and had no effect on brain water or hemoglobin contents.
Intracerebral hemorrhage most commonly affects the basal ganglia, as mimicked by our mouse model, and is well known to result in significant sensorimotor deficits, as well as a drastic deterioration in learning and memory capabilities [5, 53]. The collagenase infusion model of ICH employed in this study is clinically significant as it mimics the spontaneous vessel rupture and the re-bleeding that occurs post ictus [36]. ICH induces both primary and secondary brain injury: primary injury mainly from the mechanical disruption and mass effect of the hematoma [4], and secondary brain injury from the metabolic products of heme/erythrocyte breakdown [60]. Secondary brain injury involves edema formation and an infiltration of inflammatory cells around the hematoma [4, 29]. The cytotoxic products of inflammation after ICH all contribute to neuronal injury and death [22]. Moreover, hematoma formation and the increased inflammation after ICH contribute to the occurrence of brain edema (increased brain water content) by reducing the integrity of the blood-brain barrier (BBB) [8, 64]. Thus, anti-inflammatory agents can alleviate BBB disruption and reverse the ICH-induced increase in BWC [9].
Fucoidan from Fucus vesiculosus has been shown to exhibit anti-inflammatory activities [15, 33, 35]; therefore, we hypothesized that treatment with this drug would provide neuroprotection after ICH by reducing inflammation. Additionally, Manne et al. [37] reported that fucoidan is a novel receptor for the platelet C-type Lectin-like receptor 2, which induces platelet aggregation upon activation. We proposed that this would result in a reduction in hematoma expansion after ICH, thus improving outcomes. We found that fucoidan treatment (at 25, 75, and 100 mg/kg) failed to attenuate neurological deficits after ICH, and had no effect on brain water content and hemorrhaging, thus making null our hypotheses.
Edible seaweeds are a rich source of dietary fiber and have been historically used in the diet and as traditional medicine, particularly in some Asian countries [7, 26, 65]. In brown seaweeds, the dietary fiber is composed of soluble polysaccharides such as alginates, fucans, and laminarans, along with insoluble fibers mainly made of cellulose. Fucans are cell wall polysaccharides that are classified into three families: fucoidans (homofucans), ascophyllans, and glycuronofuco-galactans sulfate. Fucoidans are fucose-containing sulfated polysaccharides (FCSPs), which consist mainly of a backbone of (1-3)- and (1-4)-linked α-L-fucopyranose residues that may be substituted with sulfate, single L-fucosyl residues, and/or short fucoside chains [3]. Research on fucoidan has increased greatly in the last decade, due to interest in its varied potentially beneficial biological activities [3]. Despite this, the mechanisms of fucoidan, and the particular structures or fractions that are responsible for different biological activities, are still unclear, owing to a lack of standardized extraction and purification protocols.
Li et al. [33] reported that fucoidan was protective in myocardial ischemia/reperfusion (I/R) injury through its anti-inflammatory properties; fucoidan treatment reduced the levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), phospho-IκB-α, nuclear factor-κB (NF-κB), and the activity of myeloperoxidase (pro-inflammatory), while increasing the expression of IL-10 (an anti-inflammatory cytokine) following myocardial I/R. It has also been shown that treatment with fucoidan attenuated experimental autoimmune myocarditis by inhibiting macrophage and CD4-positive T-cell infiltration into the myocardium, thus exhibiting its anti-inflammatory actions [55]. Additionally, the anti-inflammatory effects of fucoidan were shown through its reduction of nitric oxide, prostaglandin E2, cyclooxygenase 2, and pro-inflammatory cytokines in lipopolysaccharide-induced BV2 microglia cells; the treatment also suppressed NF-κB activation and downregulation of ERK, JNK, p38 MAPK, and AKT pathways [46]. The regulation of the NF-κB signaling pathway by fucoidan was shown to be protective against diabetic nephropathy both in vivo and in vitro as well [59]. Furthermore, Raghavendran et al. [50] reported that pretreatment with fucoidan provided protection against aspirin-induced gastric mucosal damage in rats through its immunomodulatory actions on interleukins, TNF-α, and interferon-γ (IFN-γ). It was against this background of significant protection by fucoidan in various models that we speculated that fucoidan would exhibit its anti-inflammatory actions after ICH and be neuroprotective. However, our results revealed an opposite effect: fucoidan failed to ameliorate deficits after ICH.
We postulate that this may be for several reasons. Firstly, the bioactive properties of fucoidan depend on its purity, sugar composition, degree of sulfation, and molecular weight [63]. Controversies exist about the therapeutic benefits of high- versus low-molecular-weight fucoidans [11, 25, 47, 65]. Generally, however, low-molecular-weight fucoidan (<10 kDa) has been shown to have more therapeutic potential than high-molecular-weight fucoidan (>18 kDa) [7, 23, 28, 62]. In this study, we used the crude version of fucoidan from Sigma Aldrich, which has a high molecular weight of 20–200 kDa. Thus, the therapeutic potential was possibly decreased.
Additionally, fucoidan from Fucus vesiculosus is known to have powerful anticoagulant activity, comparable to heparin, a sulfated glycosaminoglycan, with which it shares a similar structure [13, 30, 31, 42], making it a part of the group of heparinoids. The anticoagulant activity of fucoidan is related to antithrombin and heparin cofactor II-mediated activity, and it has been investigated as a possible replacement therapy for heparin clinically [21, 39, 43]. Importantly, it was demonstrated that higher-molecular-weight FCSPs (28 kDa and 50 kDa) have higher anticoagulant activity than lower-molecular-weight FCSPs (10 kDa) [45]. In fact, cleavage of fucans by only a small amount greatly reduced their effect on thrombin inactivation mediated by heparin cofactor II [48]. These data suggest that the high-molecular-weight fucoidan may have been exerting its anticoagulant actions in our ICH model, and this accounts for our finding that there was no reduction in hemoglobin content with treatment. The hemoglobin assay we utilized measures the absorbance of hemoglobin as a quantification of ICH and can be used to evaluate the effectiveness of potential thrombolytic or anticoagulant therapies [12]. Because our initial hypothesis was that fucoidan might reduce hemorrhaging via its activity on the platelet receptor CLEC-2 [37], we conducted the hemoglobin assay to test this outcome. Based on our contrasting findings, we postulate that the anticoagulant effect of crude fucoidan may be stronger than its potential procoagulant ability, especially because of its larger size, and/or that fucoidan exhibits its effects through various other pathways. It has been established that platelet CLEC-2 activates spleen tyrosine kinase (Syk), lymphocyte cystolic protein 2 (SLP-76), and phospholipase C (PLC)-γ downstream [6, 40, 54], while fucoidan has been shown to activate other downstream signaling pathways, including inhibition of the PI3K-Akt-mTOR pathway [32, 46, 57] and caspase and ERK pathways [1, 34], indicating that it functions via another receptor(s). L-selectin and P-selectin are known to be receptors for fucoidan [19], but are not defined as the only receptors for the polysaccharide, as inhibition of L-selectin did not reverse the inhibitory effect of fucoidan on proliferation, suggesting this occurred via activation of another receptor [1]. Alternatively, Zhang and colleagues [63] conducted a study evaluating the structure-activity relationship of the pro- and anticoagulant activity of fucoidan from Fucus vesiculosus and found that the polysaccharide could be pro- or anticoagulant, depending on its charge density, molecular weight, and sugar composition. Hence, in our report, fucoidan’s anticoagulant effects seem to outweigh its potential procoagulant activity. This, interestingly, coincides with a study by Cheng and colleagues [10], in which they found that the extravascular effects of thrombin, which is known to exacerbate ischemic injury and is a possible target for the prevention of hemorrhagic transformation (owing to its role in hemostasis), resulted in ICH, implying that this effect could outweigh the normal hemostatic effects of thrombin.
Another reason we propose that fucoidan treatment was not neuroprotective in our study is that it has been shown to have proapoptotic effects in various cancers [1, 2, 27, 61]. In colon cancer cells, fucoidan was reported to attenuate the levels of the X-linked inhibitor of apoptosis protein and survivin, which both play a role in cell survival [27]. Treatment with fucoidan also enhanced mitochondrial membrane permeability, and increased levels of Bak and truncated Bid, as well as tumor necrosis factor-related apoptosis-inducing ligand, in these cells, all of which are signals for cell death [27]. In human breast cancer cell lines, fucoidan treatment increased the expression of truncated Bid, and induced activation of caspases 7, 8, and 9, along with other cell death mechanisms [61]. Further, crude fucoidan was shown to reduce cell viability in lung carcinoma and melanoma cells, and to activate natural killer cell activity in mice in vivo [2]. These data suggest that fucoidan could have enhanced the apoptotic pathways occurring after ICH, increasing cell death. The heightened loss of neurons could then account for the significant neurological deficits (including the reduction in learning and memory capabilities) and the lack of amelioration with fucoidan treatment. Conversely, it has been reported that low-molecular-weight fucoidan (at 50 and 100 mg/kg/day for 3 months) alleviated cardiac dysfunction in diabetic cardiomyopathy in rats by reducing reactive oxygen species production and cardiomyocyte apoptosis [62]. Low-molecular-weight fucoidan also ameliorated injuries from renal I/R via inhibition of the MAPK signaling pathway, which resulted in reduced ratios of Bax/Bcl2 and cleaved caspase-3/caspase-3 [7]. Moreover, Li et al. [34] found that fucoidan protected ARPE-19 cells from glucose-induced oxidative stress and apoptosis through a Ca2+-dependent ERK signaling pathway. This coincides with our previous discussion that our treatment was ineffective against ICH due largely to its higher molecular weight. However, these conflicting results on the effects of fucoidan treatment also highlight the fact that the mechanisms of its activity are still not completely understood, and more studies are needed on the molecular weights and particular fractions of fucoidan that may be most beneficial in the varying pathologies.
Our study has several limitations. Several groups have treated with fucoidan chronically (over several days) and found improvements in outcomes [7, 62]. It would therefore be prudent to examine whether chronic fucoidan treatment may be neuroprotective after ICH. Additionally, it may be beneficial to fractionate the crude fucoidan and examine the effects of the various fractions on outcomes after ICH. Finally, another approach to the study would be to investigate the effects of low-molecular-weight fucoidan on ICH-induced inflammation, edema, hematoma expansion, and neurological deficits.
Conclusion
We examined the effects of fucoidan from Fucus vesiculosus on brain water content, hemorrhaging, and neurological outcomes after ICH. Our findings revealed that fucoidan did not reduce brain water or hemoglobin contents after ICH, nor did it attenuate neurological deficits. Although there have been various studies highlighting the protective effects of fucoidan through its anti-inflammatory activities, we conclude that our findings were contradicting because of the use of crude fucoidan, which has a high molecular weight and may be proapoptotic and anticoagulant, essentially negating any anti-inflammatory effects. Low-molecular-weight fucoidan has shown greater therapeutic efficacy. Additionally, there are various fractions of fucoidan and multiple ways of fractionating, making it hard to differentiate the effects of this treatment and highlighting the need for more studies to determine which fraction of fucoidan shows the most therapeutic potential.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded by National Institutes of Health grant NS082184 to JHZ and JT.
References
1.
2.
Ale MT, Maruyama H, Tamauchi H, Mikkelsen JD, Meyer AS (2011) Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int J Biol Macromol 49:331–336CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








