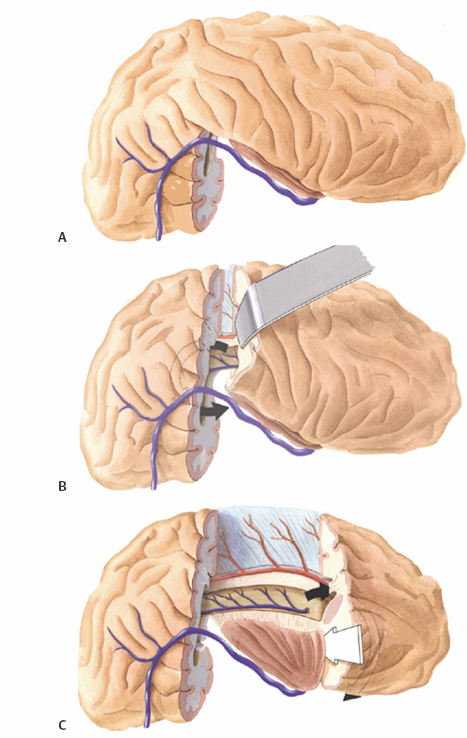
15 Functional Hemispherectomy and Periinsular Hemispherotomy The introduction of functional techniques in surgery for hemispheric epilepsy has generated renewed interest in this small subgroup of epilepsy surgery. Although anatomical hemispherectomy for the treatment of intractable epilepsy secondary to widespread hemispheric damage provided excellent seizure control, this technique introduced by Dandy,1 but first used by McKenzie to control seizures,2 and later on popularized by Krynauw,3 was abandoned following the observations of many complications, some occurring late, namely superficial cerebral hemosiderosis.4 The observation that subtotal hemispheric removal led to fewer postoperative complications, although at the expense of reduced seizure control, led Rasmussen to introduce functional hemispherectomy, consisting in subtotal hemispheric removal, but complete disconnection; the aim was to lower complications while providing seizure control similar to anatomical hemispherectomy.5,6 The objectives of functional hemispherectomy were met as demonstrated since its introduction more than 30 years ago; actually, functional hemispherectomy has been associated with fewer complications and good to excellent seizure outcome.7 The technique of functional hemispherectomy evolved over time to less brain resection in favor of a greater ratio of disconnection, leading to the technique of periinsular hemispherotomy (PIH) first reported in 1993.8 These disconnection techniques that minimize brain resection and maximize disconnection are essentially of two types. The lateral approaches include functional hemispherectomy first described by Rasmussen5 and PIH described in details in 19959; variants of this approach have also been described.10,11 The other approach is the vertical hemispherotomy described by Delalande et al.12 All these techniques aim at reducing perioperative morbidity and long-term complications, while maintaining the same seizure outcome that would be otherwise obtained with anatomical hemispherectomy. The indications for surgery in the setting of hemispheric epilepsy have similarities to that of epilepsy in general, but have a few specific differences.13 Because most patients with hemispheric epilepsy are in the pediatric age group, the noxious effects of seizures and multiple drugs on the developing brain need to be considered and weighed against the risks associated with surgery at a young age. Patients considered for hemispheric epilepsy should fulfill certain absolute criteria: intractable epilepsy usually established early and contralateral loss of voluntary distal motor function. Neuropsychological examination to establish baseline functioning and the integrity of the normal hemisphere to support most cerebral functions is essential. The preoperative investigations should include magnetic resonance imaging (MRI) and electroencephalographic (EEG) studies that should be concordant and demonstrate widespread abnormalities involving the whole hemisphere contralateral to the hemiplegia. EEG abnormalities ipsilateral to the good hemisphere, which are quite frequent findings in this population, are not an absolute contraindication to surgery.14 Hemispherectomy patients are subdivided into certain distinct surgically remediable hemispheric epilepsy syndromes. The congenital syndromes include infantile hemiplegia seizure syndrome, hemimegalencephaly, Sturge-Weber syndrome and nonhypertrophic migrational disorders. The most common and well-recognized acquired syndrome is Rasmussen’s encephalitis; other acquired causes also include sequelae of trauma, hemorrhagic lesions, and meningoencephalitis. The timing of surgery is a matter of debate. Although the presence of a complete hemispheric syndrome, that is, hemiplegia, hemianopsia, and brain hemiatrophy, are considered necessary for the indication of hemispherectomy, we are of the opinion that, at least in children, earlier surgery is preferable because the window of opportunity offered while the plasticity of the brain remains high should not be lost. Furthermore we strongly believe that, in certain hemispheric epilepsy syndromes that are progressive and invariably lead to a complete hemispheric deficit (Rasmussen’s encephalitis and extensive Sturge-Weber syndrome), hemispherectomy should be performed prior to maximal deficit. It remains that each clinical situation should be individualized. General anesthesia is used for the procedure. The patient is placed in the supine position, a soft cushion under the ipsilateral shoulder, with the head fixed on a three-pin fixation system, or taped, turned 90 degrees to the contralateral side, and minimally extended. A large “U”-shaped skin incision and flap are used with the anterior limb originating at the zygoma, overlapping the coronal suture and extending medially to reach the midline; it goes posteriorly along the midline to the lambdoid suture and extends inferiorly toward the transverse sinus 2 cm behind the level of the ear. The bone flap should stop at 1 cm lateral to the midline to avoid the sagittal sinus; in cases of severe atrophy, the midline may be shifted to the ipsilateral side and thus, the landmarks may be different. The dura is reflected toward the midline. Inspection of the brain is essential to recognize a large atrophic lesion with porencephaly or cystic replacement of portions of white matter, shift of structures due to atrophy and consequent bone remodelling, and venous and arterial structures to be preserved. Palpation should indicate whether the resection-disconnection will be performed with the manual suction-aspiration technique or with the ultrasonic aspirator. The aim of surgery consists in anatomical subtotal removal, but complete disconnection of the hemisphere. There are four main surgical steps to functional hemispherectomy: (1) excision of the temporal lobe (including amygdala and hippocampus); (2) excision of the frontoparietal region (cortex and white matter), including the parasagittal tissue down to the corpus callosum (CC); (3) interrupting the frontal and parietooccipital fibers left entering the CC at their junction with the CC; and (4) resecting the insular cortex. The anterior temporal lobectomy should reach posteriorly to a plane corresponding to the anterior portion of the ventricular atrium. This will facilitate the posterior disconnection of the parietooccipital lobe in a later step (Fig. 15.1A). The resection is performed according to standard subpial resection technique. The pia overlying the first temporal gyrus (T1) is coagulated and incised. T1 is excised with aspiration until the circular sulcus is encountered; the inferior portion of the insula is thus exposed. A posterior incision is created with similar technique, going from the posterior T1 to reach the floor of the middle fossa. The collateral sulcus is exposed from this lateral approach, and the white matter between the inferior insula and the top of the collateral sulcus is resected until the temporal horn is entered. The resection of the temporal lobe is continued by section of the infrainsular white matter, using the bipolar coagulation, with one limb in the ventricle and the other just inferior to the insula. The anterior temporal lobe is freed by subpial aspiration, prolonging more anteriorly and medially the subpial resection of T1, until the free edge of the tentorium is identified. The incision is prolonged lateral to the hippocampus and parahippocampal gyrus to reach the posterior section; the temporal neocortex is excised. The medial structures are then excised, starting with the uncus and amygdala; the amygdala should be resected rostrally until a plane corresponding to the roof of the temporal horn, and medially as long as there is pia visualized. The hippocampus is resected using suction, with great care to preserve the medial pia. During this step, large sylvian veins as well as the vein of Labbé should be preserved. Passing arteries going to the posterior temporal and anterior occipital lobes should also be preserved. The central region volume to resect extends anteriorly from the level of sphenoid wing toward the midline in a plane underlying the coronal suture; posteriorly the incision level will extend from the posterior temporal resection site to the midline just anterior to the lamda. There are four stages for the central region removal. Initially, the frontoparietal opercular cortices are resected according to the subpial aspiration technique. In the frontal region, this starts at the level opposite the sphenoid wing, and extends posteriorly to a level corresponding to the most posterior T1 resection. The suprasylvian portion of the insula is exposed until the circular sulcus is reached. The white matter of the corona radiata is transected perpendicularly, from front to back, to reach the lateral ventricle. In a second stage, a cortical incision reaching the ventricle is made in the parietal lobe corresponding to the posterior margin of the frontoparietal slab to be removed; it extends from the most posterior part of T1 resection, to the falx, just anterior to the lambda, and reaches the CC parasagittally (Fig. 15.1B). In a third stage, a frontal incision is performed from the pterional area through the whole thickness of the frontal lobe reaching slightly posterior to the sphenoid wing, entering the frontal horn of the ventricle and extending also along the falx to reach the CC. Identification of the cingulate sulcus, as well as the parasagittal pia and the A2 segment of the anterior cerebral artery are good anatomical landmarks for orientation. Finally, the central resection is completed with dissection of the parasagittal tissue, either subpially or extrapially, until the fibers entering in the boby of the CC are transected. The central region brain volume can be resected (Fig. 15.1C). Despite a large resection of the frontoparietal tissue extending to the CC, the medial portions of the frontal and parietal lobes are left attached to the CC. The callosotomy has to be completed by sectioning all fibers entering the CC at the level of the genu and rostrum anteriorly, as well posteriorly at the level of the splenium. This is accomplished from within the ventricle, transecting tissue on the medial wall, as it reaches the CC; visualization of the medial pia and pericallosal vessels are good landmarks to guarantee the disconnection (Fig. 15.2A).
Indications
Operative Techniques
Functional Hemispherectomy
Temporal Lobectomy
Resection of the Frontoparietal Region
Frontal and Parietooccipital Disconnection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








