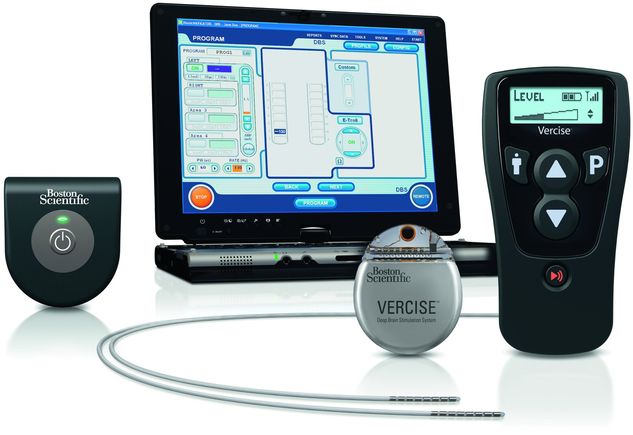
Deep brain stimulation (DBS) system, including a neurostimulator, extension, and DBS lead.

Patient and clinician programmers.
Neurostimulator
The neurostimulator is a titanium unit containing the electronics and power supply of the DBS system (Figure 6.3a,b). It is implanted in a subcutaneous pocket, usually located under the clavicle but alternatively in the abdomen. In the United States, Medtronic, Inc. has been the sole manufacturer of FDA-approved DBS systems since 1997. Their current portfolio of DBS neurostimulators in the United States and worldwide includes the Activa SC, a single-channel, primary cell (non-rechargeable) model; the Activa PC, a dual-channel primary cell model; and the Activa RC, a dual-channel rechargeable model. In Europe, additional neurostimulators have recently become available. St. Jude Medical (SJM) provides the Libra and Libra XP, primary cell models with single and dual channels, respectively. They also have the rechargeable dual-channel Brio. The latest company to receive CE Mark approval from the EU is Boston Scientific Corporation (BSC), for their dual-channel rechargeable Vercise DBS system.

Single-channel neurostimulators
These devices provide stimulation to one DBS lead, treating a target in one hemisphere of the brain. Patients requiring bilateral DBS treatment, and therefore two DBS leads, will thus need two single-channel neurostimulators. Usually one is placed under each clavicle. Intuitively, it may seem advantageous to have only one (dual-channel) neurostimulator, but there are some potential advantages to using two separate devices in a patient. The smaller size of the single-channel device may be more comfortable for small or thin patients than a dual-channel primary cell device. Further, if the device fails or needs to be removed due to infection, patients who originally had two neurostimulators can receive at least some benefit from the remaining contralateral neurostimulator.
Dual-channel neurostimulators
Dual-channel neurostimulators deliver stimulation to two separate DBS leads, enabling bilateral therapy. Implanting only one neurostimulator, rather than two, may be surgically advantageous, since fewer incisions and less tunneling of extensions are required. In order to provide sufficient power, however, a larger primary (non-rechargeable) battery within the neurostimulator is required, thus resulting in a larger size device. The rechargeable dual-channel neurostimulator addresses this issue by providing bilateral stimulation in a compact device. In order to achieve the small device size and prolonged battery longevity afforded by a rechargeable system, patients using such a device are required to recharge their neurostimulator battery on a regular (generally weekly) basis for the life of the device. This requirement may not be well suited for patients with cognitive or motor impairment or those without access to caregiver assistance. Because primary cell devices do not require maintenance by the patient, they are better suited to patients with these issues or in those who simply want a maintenance-free experience. Most dual-channel devices (Activa PC, Activa RC, Libra XP, Brio) require that the same rate of stimulation be used for each hemisphere; this is generally not a clinically meaningful limitation, but occasionally patients may benefit from the ability to assign different stimulation rates on each side to optimize symptom control. With the Vercise system, on the other hand, different rates of stimulation can be used for each DBS lead.
Quadripolar DBS leads
Presently in the United States two different DBS leads are available for use in movement disorders (Figure 6.4). The leads contain four platinum/iridium electrodes encased in a polymer-based insulation. Both of the leads (Medtronic 3387 and 3389) measure 1.27 mm in diameter and have individual electrodes that are 1.5 mm in length. The difference between these two lead models is due to the spacing between each of the four electrodes, with one model (Medtronic model 3387) having inter-electrode spacing of 1.5 mm (providing a total span of 10.5 mm with which to stimulate) and the other (Medtronic model 3389) having 0.5 mm inter-electrode spacing (providing a total span of 7.5 mm). Either lead can be used for stimulation in the usual targets to treat movement disorders, but often the lead with the wider span is used for larger targets (ventral intermediate nucleus of the thalamus [Vim] or globus pallidus internus [GPi]) and the lead with the smaller span for the smaller target (subthalamic nucleus [STN]). The model 3387 lead is typically used for stimulation of the anterior nucleus of the thalamus (ANT) for epilepsy. A third lead (Medtronic model 3391) is available for stimulation of the target used to treat obsessive-compulsive disorder, the anterior limb of the internal capsule/ventral capsule/ventral striatum; this lead has electrodes measuring 3.0 mm each, with 4.0 mm spacing between the electrodes.
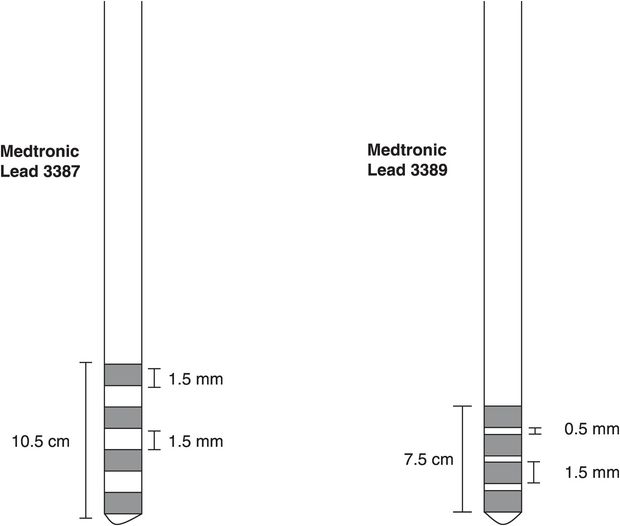
Deep brain stimulation leads (quadripolar).
Available in Europe and certain other countries, the two SJM leads are very similar, with 4 electrodes of 1.5 mm and inter-electrode spacing of 0.5 mm or 1.5 mm. The main difference is that the bottom (ventral) electrode is 3 mm. The BSC lead is essentially different from the others in that it consists of eight electrode contacts rather than four. The dimensions are otherwise the same, with electrodes of 1.5 mm and inter-electrode spaces of 0.5 mm.
Extensions
The extension, a small-diameter wire cable encased in silicone insulation, is used to connect each DBS lead to the neurostimulator (Figure 6.1). The extension, which is tunneled under the skin, attaches to the DBS lead via a connector that is usually placed above the mastoid region. Extensions are available in different lengths to accommodate different body sizes and placement of the neurostimulator in either the chest or abdominal area.
Clinician programmer
This portable device (for example, Medtronic model 8840, Figure 6.2; BSC, Figure 6.5) is used by clinicians to communicate wirelessly with the neurostimulator using radio-frequency telemetry. Using a programming wand, the clinician uses the programmer to stipulate electrode configurations, to adjust the various stimulation parameters, and to activate various features of the DBS system.
Patient programmers
These instruments serve as portable “remote controls” to be used by patients and their caregivers to interact with their neurostimulators (Figures 6.2 and 6.5). Depending on the model of the neurostimulator and its associated patient programmer, patients may be able to turn their device on and off, check the battery status of their device, adjust stimulation parameter levels within clinician-specified ranges, select from among different groups of stimulation settings, and (for a rechargeable neurostimulator) ascertain the battery charge status.
Adjustability of DBS
In order to optimize the effects of DBS by providing maximal symptom suppression without unacceptable stimulation-induced adverse effects, clinicians can modify the electrode configuration and the electrical parameters used to deliver stimulation. Elements of electrode configuration include designating which electrode(s) on the DBS system are active and the arrangement of electrode polarity (assigning the negative and positive electrodes; Figure 6.6). Stimulation parameters that can be adjusted include the amplitude, pulse width, and rate (Figure 6.7).
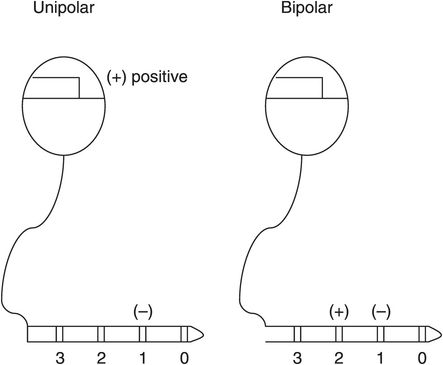
Example of simple unipolar and bipolar electrode configurations on deep brain stimulation leads.
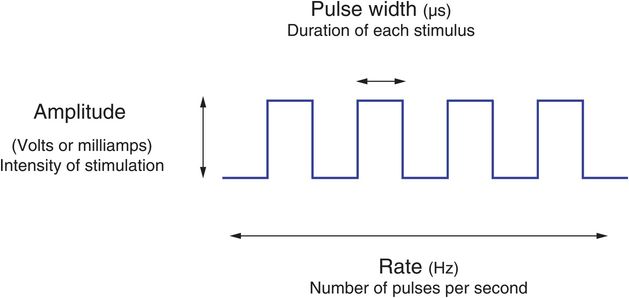
Stimulation parameters for deep brain stimulation.
Electrode configuration
There are four electrodes per lead (and eight with the BSC system) that can be used as the active electrode, alone or in combination. The clinician determines which electrode(s) provides the best relief of symptoms (as described below) to the patient, with the active electrode producing clinical benefit assigned to have a negative polarity (that is, to serve as the cathode).
In addition to selecting the negative electrode(s), the clinician also has a choice designating the positive electrode(s) (the anode; Figure 6.6). With unipolar (also known as monopolar) stimulation, the metal case of the neurostimulator is designated as the positive electrode. With bipolar stimulation, the case is not active; instead, one (or more) of the remaining electrodes on the DBS lead serves as the positive electrode. Unipolar stimulation creates a spherical area of stimulation around the negative electrode, whereas bipolar stimulation creates a more elongated but constricted field between the two selected electrodes. The clinician can take advantage of the more focused stimulation of the bipolar configuration to limit spread of the field and therefore reduce side effects of stimulating structures adjacent to the intended stimulation target.
Amplitude
The amplitude is the main parameter used to control the intensity of stimulation.
Increasing the amplitude will cause further spread of the field of stimulation, affecting neural elements at increasing distances from the electrode. It is estimated that at usual settings the electrical current diffuses about 3 mm radially from the electrode. Historically, DBS devices have operated as constant-voltage devices, in which the amplitude of stimulation is controlled by adjusting voltage. The voltage can be adjusted in small increments (usually in 0.1 volt (V) steps), between 0 and 10.5 V. Newer devices (Activa RC and Activa SC and PC, Medtronic, Inc.) can also be used in a constant-current mode, in which the amplitude is regulated by adjusting the current, with the ability to set the amplitude between 0 and 25.5 milliamps (mA). Additional devices operate exclusively in the constant current mode (Libra, Libra XP, Brio, and Vercise).
Pulse width
Pulse width refers to the duration of each electrical pulse delivered.
Increasing the pulse width will excite more and different neuronal elements within the same volume of tissue for a longer period of time. Usually a pulse width of 60 or 90 microseconds (μs) is chosen initially, and increased if needed for optimal symptom control (with the maximum pulse width being 450 μs). Pulse widths shorter than 60 μs can be obtained with the Vercise system (minimum 10 μs). Employing lower pulse widths may be of strategic use when encountering stimulation-induced adverse effects.
Rate
In general, the efficacy of DBS is based on high frequency stimulation. Rates (or frequencies) below 50 pulses per second (pps) or Hertz (Hz) are typically not efficacious for most conditions with stimulation of typical brain targets and can actually cause stimulation-induced worsening of tremor and other symptoms in movement disorder patients.
In most patients with PD and ET, the rate of stimulation is usually ≥ 130 Hz. In many instances, further rate increases will not offer substantial additional benefit.
A minority of patients, however, will respond to higher rates of stimulation, especially in the case of recalcitrant tremor. The maximal rate that can be programmed varies with the device, between 185 and 250 Hz. Most commonly used rates are between 130 and 185 Hz for PD and ET, whereas the situation may be more variable for dystonia, with some patients responding to lower rates (50–60 Hz) of stimulation (see Chapter 9).
Initial visit (Table 6.1)
Before visit
Review postoperative imaging
Review intraoperative records
Contact patient with instructions
At visit
Before programming
Take interval history
Surgical side effects
cognition, mood, micro-lesion effect
Motor symptoms
Dyskinesia exacerbation (for PD patients)
Medication changes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree