30 Fusiform Intracranial Aneurysms
Management Strategies
Introduction
In reviewing treatises of the past decade1,2 on this topic, it is clear that neuroimaging represents major progress in the understanding of these lesions. The contributions of rotational digital subtraction angiography (R-DSA), computed tomographic angiography (CT-A), and MRI have refined the preoperative understanding of this vascular lesion and further clarified the interaction with the surrounding parenchyma. While diagnostic radiology provides an improved ability to formulate a surgical plan, the rapid evolution of endovascular techniques has changed the role of the cerebrovascular surgeon in the management of fusiform intracranial aneurysms. Endovascular vessel reconstruction and flow diversion techniques have, in many cases, provided a viable alternative in the management of fusiform aneurysms.3–6 Despite these improvements, many such lesions are “beyond the reach” of endovascular devices and encourage cooperation between surgical and endovascular specialists to execute the best possible management of this complex disease.7–11 To be part of this multidisciplinary team, the modern cerebrovascular surgeon must be able to demonstrate proficiency in the use of intracranial bypass techniques.
Preoperative understanding
Potential Causes
Although many factors may contribute to their development, fusiform aneurysms are generally suspicious for a dissecting etiology that may or may not have an atherosclerotic component.12–14 The presence of intramural hemorrhage has been observed in both acute and chronic lesions.14 The contribution of infection, heritable arteriopathy, neoplasia, radiation, or previous cerebrovascular surgery should be considered when formulating a treatment strategy.1,12,15–18 The location of the aneurysm may also suggest the etiology of the aneurysm and modify the management strategy. For example, fusiform aneurysms of the distal anterior circulation aneurysms are more likely to be related to trauma or infection, while a fusiform aneurysm of the vertebrobasilar junction frequently has an atherosclerotic component.
Trial Balloon Occlusion (TBO)
The use of trial balloon occlusion (TBO) should be a routine consideration in the management of all fusiform aneurysms. The substantial pathology of the arterial wall increases the possibility of permanent wall injury and need for parent vessel occlusion as a salvage during the procedure. In many institutions, the necessity and type of bypass (high-, mid-, low-flow) are determined by information obtained from the results of a protocol including this technique.19 Although 80% of patients may tolerate ICA or nondominant vertebral artery occlusion,20–23 a 20% chance of morbidity is unacceptable. Our technique of utilizing clinical examination and single photon emission computed tomography (SPECT) has been previously published.24 Since the introduction of the balloon occlusion technique by Matas,25 significant changes in endovascular technology have allowed safer and more precise placement of a compliant balloon in an area that more closely mimics clip placement. This precise placement has produced more reliable results. Anecdotal experiences of balloon occlusion of the inflow to fusiform aneurysms have been discussed (personal communication) but not reported. The use of provocative measures such as EEG and hypotension in an attempt to further improve predictive values has been reported with up to a 95% correlation with successful vessel sacrifice.19,26–28
Surgical considerations by location
Middle Cerebral Artery
Fusiform aneurysms in the MCA have an uncertain natural history. In addition to being a common location for rupture, a concurrent risk for ischemia and symptoms attributed to mass effect also exists. Although data from the International Study of Unruptured Intracranial Aneurysms (ISUIA) noted a 5-year cumulative risk of 40% in giant lesions of the anterior circulation,29 as an isolated group, the relative risk of rupture for the middle cerebral artery appears to be inversely proportional to growth. In a retrospective meta-analysis of spontaneous fusiform aneurysms of the middle cerebral artery, Day et al. emphasized that the risk of ischemia and mass effect was higher in the larger lesions than the risk of rupture.15 As a group these lesions are exposed more superficially, are less likely to be endovascular candidates, and are frequently treated by primary reconstruction.30 When bypass options are entertained, the strategy should minimize the number of vascular territories at risk even if a staged procedure is necessary. Reliance on collateral circulation in this location is a gamble for the patient.
Case 1: Calcified lesion of proximal M2
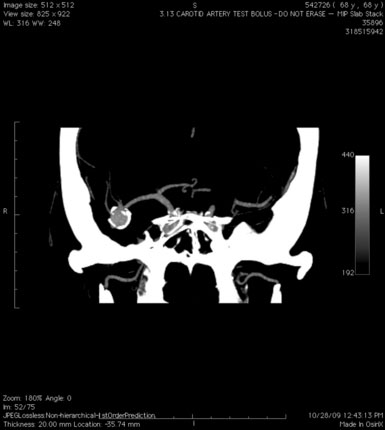
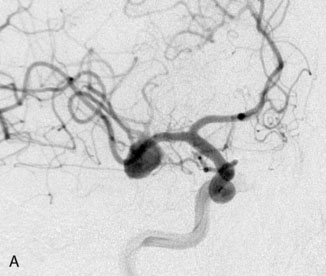
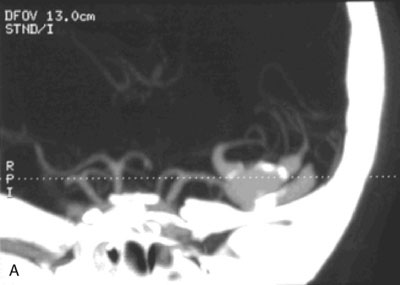
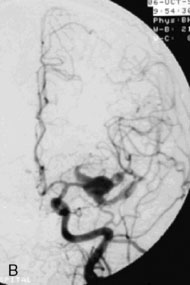
A 55-year-old male presented with a 3-month history of progressive left hand numbness. MRI and MRA suggested an 18 × 14 × 11 mm fusiform, right middle cerebral artery aneurysm that involved the origin of the insular M2 branch as well as subsequent exiting M3 branches. These findings were confirmed by CTA, which further clarified significant mural calcifications (Figure 30–1). Both CTA and R-DSA (Figure 30–2) made it clear that the multiple M3 vessels originating from the dome of the aneurysm would require the use of at least two bypass conduits to maintain the patency of these vessels if the aneurysm was to be obliterated.
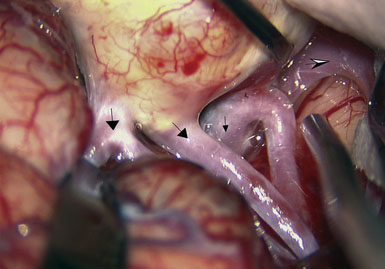
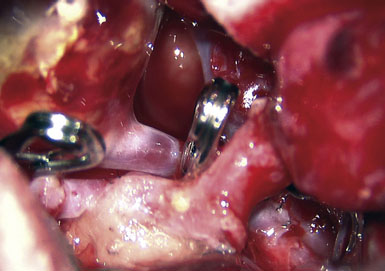
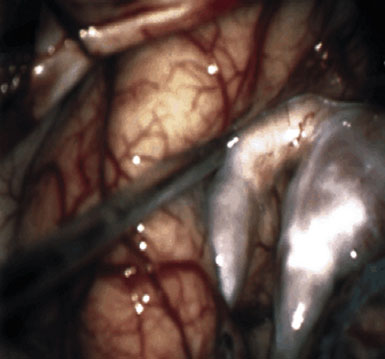
Preoperatively, a plan was made for the use of the ipsilateral superficial temporal artery (STA) and lesser saphenous vein as possible bypass conduits. The use of both STA branches was also a consideration. Intraoperative evaluation of the aneurysm confirmed the radiographic anatomy (Figure 30–3). The close proximity of a large anterior temporal artery made it possible to include an in situ, side-to-side anastomosis to an M3 vessel with the treatment options. A cut-flow of 20 cc/min was measured in the frontal branch of the STA. This was thought to be more than adequate to supplement ultrasonic flow measurements of 13 cc/min (Charbel microflow probe, Transonic Systems) noted at the medial M3 trunk. The side-to-side anastomosis was chosen for the lateral M3 branch (Figure 30–4) due to the inadequate length of the parietal STA branch.
After establishing patency of the bypassed segments, primary clipping of the aneurysm was prevented by the mural calcifications. The aneurysm was opened and tandem clips were placed for complete occlusion that was confirmed by postoperative angiography (Figure 30–5).
Case 2: Metastatic cardiac myxoma of the M1 bifurcation
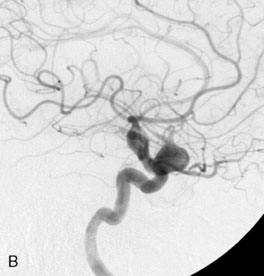
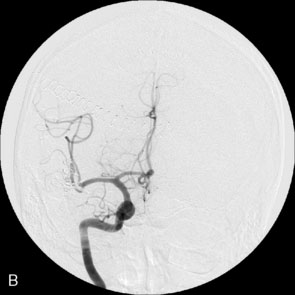
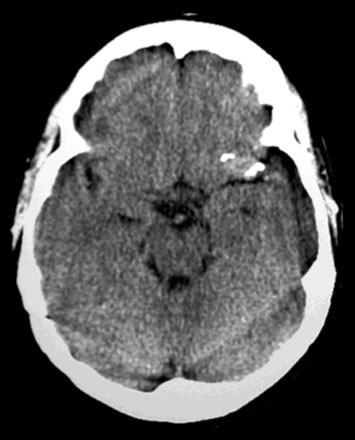
A 34-year-old, right-handed woman presented with episodic left hemisphere transient ischemic attacks (TIAs). A previous “stroke” at age 4 was followed by an operation on her heart for treatment of atrial myxoma. Evidence of previous ischemia and calcifications were visualized in the left sylvian fissure (Figure 30–6). Coronal CT reconstructions revealed the partially calcified wall of the lesion (Figure 30–7A). Angiography demonstrated a large, left-sided fusiform aneurysm involving a distal M1 segment and all exiting M2 segments. The proximal M1 segment containing the origins of the lateral lenticulostriate vessels was unaffected (Figure 30–7B).
With the likelihood of metastatic cardiac myxoma, a complete excision of the distal middle cerebral artery and proximal M2 vessels was planned via a left frontotemporal craniotomy. A right radial artery was harvested to serve as the conduit for the EC-IC bypass from the external carotid artery. After complete exposure of the left MCA from the origin of lateral lenticulostriate vessels to distal normal M2 branches (Figure 30–8 and Figure 30–9A), one M2 was amputated from the aneurysm and sutured, end to side, to the radial artery graft 2 cm from its distal tip (Figure 30–9B, 1). A temporary clip was placed on the radial graft distal to this anastomosis and the second M2 insular branch was amputated and sutured to the distal tip of the radial artery graft (Figure 30–9B, 2). Then temporary clips were placed on the M1 segment distal to the origin of the lenticulostriate vessels and a second clip on the third M2, just distal to its origin. The aneurysm was then excised and removed from the field. A short radial artery interposition graft was placed (end to end) between the M1 and remaining M2 (Figure 30–9B, 3).
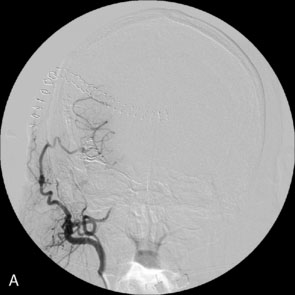
Immediate postoperative angiography demonstrated opacification of the two insular M2 segments via the EC-IC bypass (Figure 30–10). Significant spasm of the radial graft was clear. The more proximal M2 segment filled in continuity with the native M1. Clinically, the patient had a brief exacerbation of her mild expressive aphasia, but had returned to her preoperative level of functioning with 3 days. No clinical changes were noted at a 5-year follow-up examination.
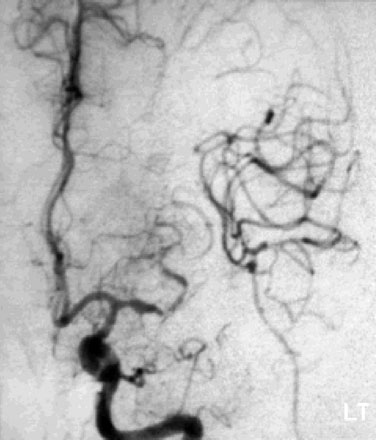
Figure 30–10 Intraoperative angiography of completed bypass with exclusion of the fusiform aneurysm.
Learning points
The MCA lesions presented here represent cases where disease pathology required complete trapping and exclusion of a necessary arterial segment. Thorough preoperative evaluation of the imaging allowed for multiple reconstructive options to be available in surgery. In Case 1, the intraoperative evaluation and blood-flow measurements (Transonic Systems) suggested robust collateral flow during proximal occlusion of the aneurysm. The ability to use a single STA to supply two exiting branches was also suggested by these flow measurements. Although the “double-barrel bypass”30 would have, similar to Case 2, put less vascular territory at risk, the orientation of the vessels exiting the calcified aneurysm would not allow for a tension-free anastomosis of the second STA. Although an in situ anastomosis was performed, preparation of the radial artery for interposition grafting might have minimized vascular risk and provided a conduit with a better size match than the saphenous vein that was available.
In Case 2, it is important to emphasize the utility of addressing vascular beds individually. The use of the cervical radial artery bypass allowed for “tying in” each bed serially such that no more than 20 minutes of occlusion was permitted per arterial territory. Previous studies have confirmed a significant increase in operative morbidity when temporary occlusion persists for longer than 19 minutes.31 The postoperative spasm of the radial artery, noted in Case 2, is a reported liability of the radial artery bypass. This occurred despite use of the pressure distension technique that has been described by Sekhar, et al.32 Endovascular angioplasty should be considered should medical blood pressure manipulation not succeed in improving the clinical exam.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree