Fig. 63.1
Henri Laborit (1914-1995). GHB discoverer and synthesizer
First Clinical Trials
These seemingly unique properties led initially to the use of GHB in the treatment of patients suffering from major depressive disorders and bipolar disorder . In both, there is severe insomnia that, in the latter, it usually precedes the periods of hypomania or mania. It was hoped that GHB would restore a normal sleep architecture, prolong the duration and continuity of sleep, correct the timing of REM sleep and increase the duration of SWS. But, in early clinical trials in patients with these conditions, GHB unexpectedly reversed the normal sequence of sleep states and radically altered the architecture of sleep [10] by further shortening the already brief REM sleep latency and even inducing sleep-onset REM sleep periods that could last close to an hour and were then followed by almost equally prolonged periods of SWS. GHB appeared, in retrospect, to have partially induced the polysomnographic (PSG) features of narcolepsy.
Discovery of GHB Efficacy in Narcolepsy
The pathogenesis of narcolepsy at that time was poorly understood, in part because the integrating functions of the hypocretins (orexins) had not as yet been discovered [11–13]. In PSG studies of narcolepsy, the orchestration of sleep/wake states appeared to have broken down and the different states of sleep and their components to have become dissociated from one another and gone adrift around the 24-h. Others had shown that cataplexy reflects the selective activation in wakefulness by emotional stimuli of the motor atonic component of REM sleep, and that sleep paralysis represents continuation of REM atonia mechanisms with those of cerebral REM sleep being replaced by wakefulness. These features had led me [14] to define narcolepsy as a disease of state boundary control.
Concerning sleep distribution across the 24-h, the then current belief was that the daytime sleep and drowsiness/sleepiness in narcolepsy-cataplexy reflected a homeostatic “make-up” of the highly fragmented and reduced amount of night sleep. Much later this was shown not to be the case, as 24-h ambulatory sleep/wake recordings in narcolepsy–cataplexy found that there was no correlation between the amount of night sleep and that of subsequent day sleep [15]. The evidence much more strongly supported a weak circadian arousal rhythm as being the cause [16].
Given GHB’s proven capacity to promote both REM and SWS, I proposed to Mortimer Mamelak that we use it to try and consolidate sleep into the night-time period. GHB in its sodium form was imported into Canada from Laboratoire Égic in France. Our open-label studies began in 1974/5 [17]. GHB was given in two or three doses with the objective to induce as continuous a night of sleep as possible. The first dose was 1.5–2.25 g (10–15 ml) at bedtime, followed by one or two further doses of 1.0–1.5 g with awakenings, if at least 2.5 h had passed since the previous dose. The total dosage ranged from 3.75 to 6.0 g corresponding on average to some 50 mg/kg. GHB alleviated daytime sleep attacks, drowsiness and sleepiness measured by the Stanford Sleepiness Scale [18] and virtually eliminated cataplexy [17, 19–21]. Night-time sleep on GHB became consolidated with increased SWS and REM sleep, a decreased number of stage shifts per 100 min for both non-REM (NREM) and REM sleep, increased REM period efficiency, shortened REM latency and decreased stage N1 drowsiness (Figs. 63.2 and 63.3). Daytime stage N1 drowsiness, sleep attacks and naps were markedly reduced. A few patients no longer had any daytime sleep on nocturnal GHB (Fig. 63.4). Some nocturnal electroencephalography (EEG) delta waves on GHB were of extreme duration of 1.5–2.0 s (Fig. 63.5) and this appeared to be a direct drug effect.
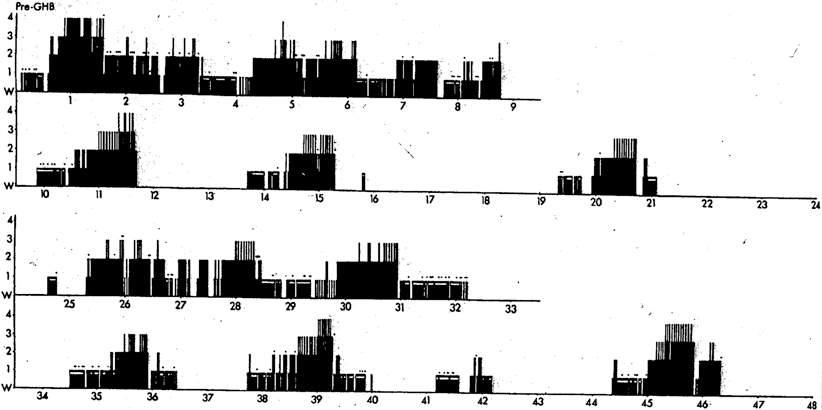
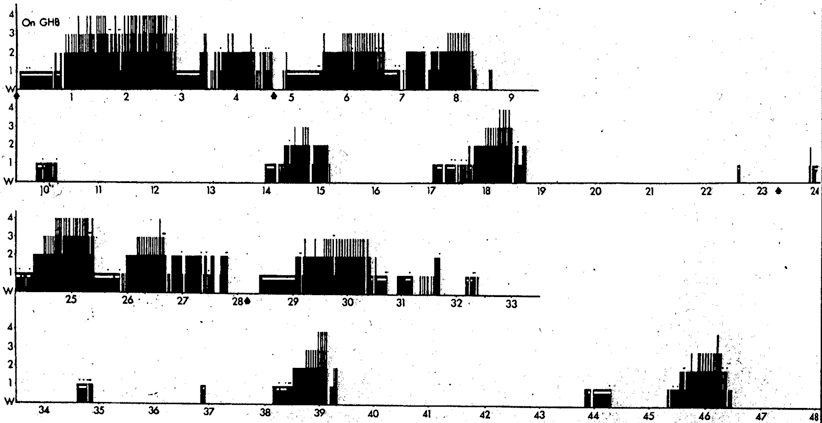
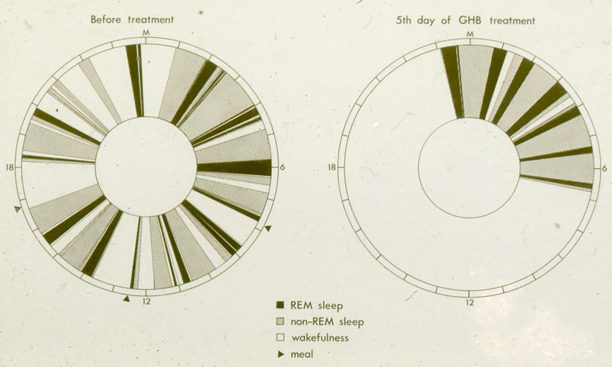
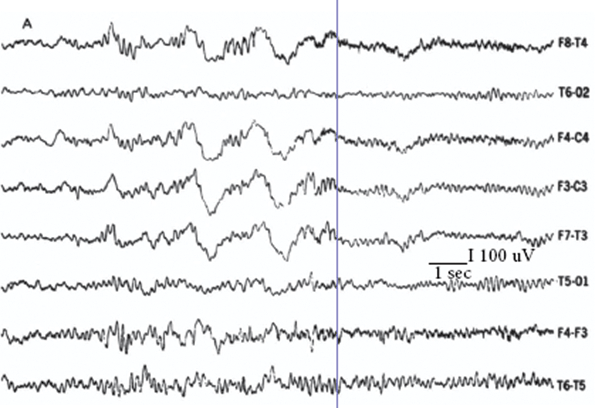

Fig. 63.2
A48-h pre-GHB baseline recording of a patient with narcolepsycataplexy shows frequent awakenings in night sleep, multiple night and day sleep onset REM sleep periods, and fragmented REM periods. Vertical axis shows NREM stages 1, 2, 3 and 4. REM sleep is shown as a white horizontal bar at the same level as NREM stage 1 (i.e., drowsiness). Major movements are shown as small triangles above the vertical stage lines. Time zero was between 10:30 and 11:00 pm in both Figs. 63.2 and 63.3 [from 21]

Fig. 63.3
On-GHB. Same patient but on days 9 and 10 of nocturnal GHB. Times of GHB administration pre-sleep and within the night sleep periods are shown as arrows below the horizontal time line. Night sleep has more SWS and is more continuous, as are the REM periods. Day sleep is greatly reduced. Patient slept on hospital bed with long wire electrodes for both studies. Ambulatory monitoring studies allowing normal movement showed less day sleep. From[21]

Fig. 63.4
24-h clocks of sleep/wake patters pre-GHB and on the 5th consecutive day of nocturnal GHB in a young patient with narcolepsy-cataplexy. GHB greatly consolidated night sleep and both REM and NREM during the nighttime sleep period, Daytime reduction of stage N1 (drowsiness), sleep attacks and voluntary naps was marked, daytime sleep being virtually abolished. Such dramatic effects were seen in only a few patients. The majority of patients showed some degree of residual drowsiness (N1) and some daytime NREM and REM sleep

Fig. 63.5
Some of the nocturnal EEG delta waves on GHB were of extreme duration of 1.5–2.0 s and this appeared to be a direct drug effect
The positive clinical effects on subjective and objective quality of night sleep, daytime improvement of alertness, reduced sleep, and suppression of cataplexy plus the other REM-based symptoms including sleep paralysis, hypnagogic hallucinations and nightmares, was seen in all patients. This was true even in four very severe patients who had not been improved on prior traditional combinations of stimulants for daytime somnolence and sleep, antidepressants for REM-based symptoms, and hypnotics for nocturnal insomnia . Tolerance did not develop in any patients on GHB and side effects were few in number and generally short-lived.
These findings of efficacy of GHB in the treatment of narcolepsy–cataplexy were soon confirmed by investigators in the USA and Europe in double-blind, placebo-controlled studies [22–24]. Other clinicians collected large case series and also found that tolerance failed to develop to GHB’s therapeutic efficacy. This was in stark contrast to the effects of the then conventional treatment using hypnotics, stimulants and antidepressants all of which too often required increasing doses to suppress the symptoms of the disease [25–27]. I have followed patients with narcolepsy-cataplexy treated with GHB for over 30 years without seeing a single patient develop tolerance to the drug.
Studies have shown that patients with narcolepsy–cataplexy, compared to normal controls, have broad negative effects on work, income, education, driving, accidents and many other parameters of quality of life [28], and that these are seen equally in North American, Asian and European patients [29]. These effects are even more pervasive and greater than those of the well-documented life effects in epilepsy patients without significant organic brain disease [30]. The treatment of narcolepsy with GHB has been shown to vastly improve the quality of patient’s lives [31].
In response to these benefits, patients with narcolepsy petitioned the orphan drug division of the Food and Drug Administration (FDA) in the USA to undertake the clinical trials required to abolish GHB’s experimental status and make it a prescription drug. Martin Scharf must be given particular credit for his persistent advocacy on behalf both of these patients and of GHB. Orphan Medical, under the remarkable leadership of its chief medical officer, William Houghton, undertook the task of bringing GHB to market. In 2002, GHB in its sodium form as sodium oxybate (Xyrem®) was approved in the USA for the treatment of cataplexy. In 2005, approval was granted for GHB in excessive daytime sleepiness (EDS) . Similar approvals for its use in EDS soon followed in Canada and Europe.
The marketing of Xyrem® on prescription began only after the completion of a series of necessary large multi-centre clinical trials in the USA, Canada and Europe. In the first of these trials [32], 136 patients with narcolepsy and cataplexy remained on their stable dose of stimulant medication and then were gradually withdrawn from their anticataplectic and hypnotic medication. Following a washout period, patients were assigned to receive either placebo or 3, 6 or 9 g of GHB for 4 weeks in equally divided doses at bedtime and again 2.5–4.0 h later. The findings revealed that the magnitude of the anticataplectic effect was dose related and was greatest in the 9-g group. This was true as well for measures of EDS that, in a number of patients, actually fell into the normal range. There was a significant decrease in the number of inadvertent sleep attacks and voluntary naps during the day in the 6- and 9-g groups, and a significant decrease in nocturnal awakenings in the 9-g group.
One hundred and eighteen of the patients who participated in the initial 4-week trial agreed to join a 12-month extension trial [33]. All patients began treatment at 6 g per night in equally divided doses, as before. Based on the clinical response, the investigators could choose to decrease the total nightly dose to 3 or 4.5 g or increase it to 7.5 or 9 g. Data from this open-label study revealed that all doses between 3 and 9 g produced significant long-term reductions in the frequency of cataplexy. This improvement was rapid and progressive during the first month of treatment; and the incidence of cataplexy fell further during the course of the year. There was also a significant reduction in daytime sleepiness for all treatment groups. Patients who were also on stimulants felt even more alert and better able to concentrate with the added use of GHB. The quality of sleep at night was significantly improved. There was no dose escalation to indicate the development of tolerance .
Another US Xyrem® multi-center study found that abrupt withdrawal of GHB from patients with narcolepsy who had received nightly doses of 3–9 g for 7 to 44 months led to a gradual return of the symptoms of the disease [34]. As in the first studies [20, 21], there was no rebound increase in cataplexy, or appearance of status catiplecticus, as often occurs in withdrawal of antidepressants [35].
The effects of GHB on sleep architecture were further examined [36] in a study on 25 patients using the Maintenance of wakefulness test (MWT) [37] and the Epworth Sleepiness Scale (ESS) [38] to determine the effects on daytime sleepiness. Subjects were maintained on their usual daytime stimulant dose. All subjects received 4.5 g of GHB each night in divided doses for 4 weeks followed by escalating doses of 6, 7.5 and 9 g at subsequent 2-week intervals. Bedtime GHB initially further reduced the already short REM latency and increased the duration of REM sleep in 4 h that followed. After 4 weeks, this effect was no longer evident and, in fact, increasing doses of GHB then progressively depressed the duration of REM sleep and increased that of SWS. Curiously, total sleep time in this study was not increased even at the highest dose, although the number of nocturnal awakenings declined significantly with increasing doses. Despite its failure to increase total sleep time, GHB showed a dose-related improvement in alertness on MWT. At the highest (9 g) dose, a number of subjects rated their sleepiness in the normal range, an effect objectively confirmed by MWT.
The beneficial effect of GHB on MWT at the 9-g dose was again demonstrated in a multi-centre placebo-controlled parallel group trial that involved 228 patients [39, 40]. The reasons for the improvement of daytime alertness on GHB have yet to be determined, but they may be related to the provision of energy to the brain by GHB or a metabolite, to its antioxidant effect or to a rebound increase in the activity of various neurotransmitter systems engaged in the induction and maintenance of wakefulness as the effects of GHB wear off in the morning [25].
Another double-blind controlled trial was undertaken on 270 patients with narcolepsy in order to compare the impact on daytime drowsiness of GHB alone with that of modafinil. GHB was studied in doses up to 9 g at bedtime [41]. MWT testing demonstrated that GHB alone was as effective in maintaining daytime alertness as was modafinil alone. But again even greater alertness was achieved when modafinil and GHB were combined, suggesting a synergistic effect. GHB alone also produced a significant decrease in the ESS rating . When GHB at night was combined with daytime modafinil, a greater reduction in the ESS occurred.
A study of the effects of GHB treatment on such psychosocial variables as level of activity, vigilance and personal productivity in 285 patients, [31] reported several very significant findings. A dose response was demonstrated with the greatest benefit found at 9 g. The authors commented that the findings were not only statistically significant but also clinically important. GHB substantially improved daytime functioning even further in the 50 % of the subjects who also received the stimulant modafinil. The levels of excessive daytime drowsiness then often fell into the normal ranges.
Narcolepsy–cataplexy and GHB in Childhood and Adolescence
The first report of GHB in childhood narcolepsy with cataplexy [42] involved a group of eight children with a severe form of the disease documented by history, nocturnal polysomnography, a modified Epworth Sleepiness Scale and the multiple sleep latency test (MSLT) [43]. On GHB all patients but one improved significantly and had marked reduction of cataplexy frequency and severity, and improved ESS ratings .
Aran et al. [44] retrospectively studied a cohort of 51 children divided into those in whom narcolepsy–cataplexy appeared before (53 %), around (29 %) or after (18 %) puberty. The only clinical difference between the three age groups was an increased occurrence of sleep paralysis with age. Weight gain was common prior to the development of the disease and puberty occurred earlier than for other family members. Streptococcus throat infections within 6 months of disease onset were reported in 20 % of the patients. PSG features were similar in all three groups. Three pre-pubertal children did not meet the usual MSLT diagnostic criteria for the diagnosis despite pathognomonic symptoms. Patients were treated with a variety of drugs including modafinil (84 %), GHB (79 %) and venlafaxine (68 %). Other drugs tried including methylphenidate, tricyclic antidepressants and SSRIs; but these were rarely continued. GHB was effective for all symptoms, modafinil for EDS and sleep attacks alone and venlafaxine for cataplexy alone. At the end of the study, half of the children were on GHB either alone or with the addition of one of the other two drugs. Older children were often maintained on more than two drugs.
Peraita-Adrados et al. [45] reported a series of nine children with narcolepsy–cataplexy treated with a variety of medications including either methylphenidate or modafinil combined with an antidepressant or, in two cases, by GHB. The children also had scheduled naps. The two children on GHB responded particularly well.
In a retrospective study of 15 paediatric patients (children and adolescents, aged 3–17 years) treated for narcolepsy–cataplexy with GHB supplemented by any prior medication for sleepiness or cataplexy [46], the supplementation of GHB improved ESS scores, reduced the number of cataplexy episodes reported by parents and reduced cataplexy severity. Three patients discontinued GHB: one for insurance reasons, another for constipation plus dissociative feelings, and the third (only temporarily) for dizziness plus body aches and pains that did not reappear after return to GHB therapy. The dosage of GHB was 5.0 ± 2.5 g per night and no tolerance developed. Lecendreux et al. [47] reported a multi-centre study that retrospectively studied 27 children treated off-label in a clinical setting. It found a good clinical and laboratory response to GHB which was well tolerated by all patients.
Unfortunately, to date there are no randomized double-blind controlled trials of GHB efficacy in narcolepsy with cataplexy during childhood or adolescence.
Obstructive Sleep Apnea Syndrome (OSAS) with Excessive Sleepiness
The positive response of EDS to GHB in narcolepsy–cataplexy led to attempts to see whether sleepiness in the more common disorder of obstructive sleep apnea would respond equally well. A main concern was that GHB at higher doses is an anaesthetic and there is a potential risk of suppression of the brainstem centres controlling respiration. The results have been mixed.
Charles George and collaborators at the University of Western Ontario have published two safety trials of the effects of GHB on respiratory function. In the first study George et al. [48] noted that OSAS often coexisted with narcolepsy–cataplexy . Sixty patients with mild-to-moderate OSA received one of four treatments: (a) 9 g of GHB in the evening, (b) 200 mg modafinil in the daytime, (c) 10 mg zolpidem before retiring or (d) placebo, as part of a randomized crossover design involving four nights with overnight PSG. The apnea-hypopnea index and the mean SaO 2 did not change significantly on GHB. Patients showed an increase in central apneas and three had significant oxygen desaturations. There were some adverse clinical effects, mainly headache and nausea. The authors concluded that in OSAS, with or without coexistent narcolepsy, GHB in some patients may cause central respiratory depression with central apneas and oxygen desaturation, and that it should therefore be used with caution.
In their second study George et al. [49] performed a 2-week assessment of GHB at 4.5 g nightly versus placebo in patients with OSAS. Compared with placebo, GHB increased reduction in the apnea–hypopnea index and also increased SWS. There was no difference in SaO2 levels, central respiratory events or in other respiratory measures. Headache was noted in a third of patients and was the only significant side effect. They concluded that short-term use of GHB at 4.5 g per night was well tolerated but that longer usage needed study, and that the drug should be used with caution in such patients. Neither study assessed the impact of GHB on daytime function.
There does appear to be some respiratory risk in this usage of GHB. Seeck-Hirschner et al. [50] reported two patients with narcolepsy-cataplexy and OSAS treated with GHB in whom there was an increase in sleep-related breathing disturbances.
Fibromyalgia and Chronic Fatigue Syndromes
Since the original report of Moldofsky [51] describing the efficacy of GHB to alleviate the characteristic widespread pain, tender points in specific anatomical regions, and non-restorative sleep of the rheumatological condition of fibromyalgia, there has been a cavalcade of confirmatory studies. Russell et al. [52] using the standard criteria of the American College of Rheumatology treated [53] a large series of 188 patients with 4.5 or 6.0 g or matching placebo once per night for 8 weeks. Patients filled in the Fibromyalgia Impact Questionnaire, the Patient Global Impression of Change, and the Jenkins Scale of Sleep of subjective sleep quality. All three scales showed improvement. GHB was well tolerated other than for occasional dose-related nausea and dizziness with both tending to resolve with continued treatment. In a study in which the majority of patients had chronic fatigue syndrome and a minority fibromyalgia, Spitzer and Broadman [54] found GHB improved 60 % those of the symptoms of pain and 75 % those of fatigue.
The first double-blind, randomized, placebo-controlled study of the efficacy of the effects of GHB on fibromyalgia was by Moldofsky et al. [55] who studied 304 patients diagnosed by the standard criteria. Polysomnography was done in 200 patients and 195 patients were randomized with 151 completing a double-blind, cross-over, placebo-controlled protocol at doses of 4.5 and 6.0 g per night. Pretreatment assessments showed high amounts of alpha intrusion in the sleep EEG (66 % of subjects), periodic leg movements in sleep (20.1 %) and moderate-to-severe obstructive sleep apnea (15.3 %). Compared to placebo, there was a significant improvement in the ESS , the Jenkins Scale of Sleep quality, the Functional Outcome of Sleep Questionnaire, the SF-36 Vitality Scale, and the Fibromyalgia Impact Questionnaire. Polysomnography showed decreased wakefulness after sleep onset with increases in stage N2 sleep, SWS (N3) and total non-REM sleep. Some side effects encountered included nausea, pain in the extremities, dizziness, restlessness and occasional urinary incontinence. Similar findings using a comparable crossover, double-blind, placebo-controlled design were reported by Russell et al. [56]. Subsequent confirmatory studies are those of Staud [57, 58], Crawford et al. [59], and an international phase 3 trial [60].
Neurodegenerative Diseases
Another promising area of GHB clinical usage is that of neurodegenerative diseases. It is now well documented that REM sleep behaviour disorder (RBD), in which the atonia of REM sleep is absent permitting complex behaviours while asleep, is often a harbinger of Parkinson disease (PD), as in some 33–65 % of cases RBD is either followed several years later by the appearance of PD or accompanies diagnosed PD [61–66]. There is a single report of GHB successfully treating RBD. The patient’s symptoms had persisted for 5 years uncontrolled on other drugs, including clonazepam . GHB at low doses led to cessation of all nocturnal events, an effect sustained during a one-year follow-up period [67].
PD has been treated with GHB with the intention of reducing the excessive sleepiness that often is a part of the disease along with the tremor, rigidity, cognitive problems and other characteristic symptoms. As reported by Arnulf and Leu-Semenescu [68], EDS in about a third of PD patients and may even be accompanied by sleep attacks without prodroma. These sleep attacks, like those of narcolepsy-cataplexy, often contain REM sleep with sleep-onset REM periods sometimes associated with hypnagogic hallucinations or frank dreams. The authors noted that modafinil is only partially effective and that patients respond much better to either GHB or anti-histamine-3 drugs. Ondo et al. also reported an improvement of EDS when PD patients are treated with GHB [69].
Stroke may also be considered a neurodegenerative disorder given the neuronal loss resulting from an ischemic or hemorrhagic event. There are no clinical reports of stroke treated by GHB. However, in an experimental study in which the middle cerebral artery was occluded [70], those mice receiving intra-peritoneal GHB (100 mg per kg, twice a day, given 8 h apart) recovered much more rapidly than those treated with saline. Recovery was assessed behaviourally by grip strength, and histologically using cresyl violet and neuronal staining. Similar results are by MacMillan [71], Ottani et al. [72] and Sadasivan et al. [73].
Alzheimer’s dementia is characterized amongst other things by oxidative stress, limited neuronal energy resources, excitotoxicity and vascular endothelial pathology. GHB has been shown experimentally to reduce the tissue damage resulting from oxidative stress. It has been proposed [74, 75] that GHB might be able to buttress this protective process which ends with the release of beta-amyloid and the formation of neurofibrillary tangles that together are the pathological hallmarks of the disease. No clinical trials have been reported.
Side Effects of GHB
GHB has repeatedly shown itself to have very few deleterious side effects. Table 63.1 summarizes those side effects reported in the already cited open-label and double-blind crossover studies in narcolepsy. Further side effects have been the focus of individual case reports. These effects are in general much more rare than those already well documented in the clinical trial studies. To date few such seemingly idiosyncratic drug reactions have been published.
Table 63.1
Side effects reported by the main clinical trials
First symptom | Second symptom | Third symptom | Fourth symptom | Other | Other, or a note | |
|---|---|---|---|---|---|---|
Thick head | Ocular discomfort | Enuresis | Confusion | Hangover | ||
Nausea with emesis | Dizziness | Weakness and fatigue | Urinary urgency | Morning sluggishness | Morning stiffness | |
Lammers [22] | Sleep paralysis + hallucination | Weight loss | Very few side effects | |||
US Xyrem® [32] | Nausea | Dizziness | Enuresis | Somnolence | Feeling drunk | Confusion |
US Xyrem® [33] | Headache | Nausea | Nasopharyngitis | Vomiting | Somnolence | |
US Xyrem® [34] | No differences from controls | |||||
Mamelak et al. [36] | Confusion if resist effects | Enuresis | Induced sleep paralysis | Sleepwalking | Weight loss | |
Xyrem® Inter [39] | Nausea | Dizziness | Enuresis | Confusion | Somnolence | Attentional problems |
Xyrem® Inter [40] | Various minor | |||||
Black and Houghton [41] | Headache | Nausea | Dizziness | Nasopharyngitis | Vomiting | Somnolence |
Husain et al. [76] reported weight loss averaging 3.4 kg in a series of 54 patients with narcolepsy being treated with GHB. Wallace et al. [53] described a patient on GHB who experienced a single instance of drug-induced sleep-driving and two episodes of sleep-eating.
Patients experiencing psychological side effects independent of clinical trials have also been reported. Rossetti et al. [77] described the occasional rapid appearance of depression when GHB was added to modafinil . Langford and Gross [78] noted that, when taken as a drug of abuse, GHB can produce serious psychiatric side effects and also withdrawal symptoms, whereas in patients taking recommended doses, such effects have never been reported. They described a single patient with narcolepsy treated with the recommended doses of GHB who showed initial altered mental status and later became psychotic on abrupt discontinuation of the medication.
In an early study, Bėdard et al. [79] found that GHB in narcolepsy–cataplexy can be associated with an increase in periodic leg movements in sleep that have the potential of opposing the sleep consolidation effect of the substance.
Extremely rarely, GHB has been reported to induce a form of parasomnia . Poli et al. [80] describe a series of narcolepsy patients treated with GHB, some with associated OSAS, in whom 14 % developed catathrenia. This condition consists of groaning and an abnormal respiratory pattern in sleep confirmed by PSG recordings. No worsening of respiratory measures occurred in the narcolepsy-cataplexy patients with OSAS, and the catathrenia was considered benign.
Four deaths have been reported in patients taking GHB. Akins et al. [81] reported a 53-year old woman with narcolepsy-cataplexy who was also taking tramadol, gabapentin, cetirizine, carisoprodol and modafinil and who had well documented significant OSAS. The combination of central nervous system (CNS) depressants and OSAS, both of which are contraindications for GHB treatment, was considered the cause of death which itself was believed to be accidental. Zvosec et al. [82] reported a further three patients who died on GHB. One was associated with GHB abuse and had post-mortem blood GHB levels that were extremely high. Concurrent use of sedative hypnotics, OSAS and obesity were in volved in the two other patients, both of whom took GHB in the recommended doses. In two of the three patients, post-mortem GHB levels were assessed and were only 141 and 110 mg/L, i.e. within the expected post-mortem range for GHB. The third case was a patient with a history of intentional drug overdose, and whose actual cause of death remained uncertain.
Zvosec et al. [83] investigated 226 deaths attributed to GHB. These included 213 from cardiorespiratory arrest and 13 fatal accidents. Seventy-one instances (34 %) had no related intoxicants. Post-mortem blood GHB was 18–4400 mg/L (median = 347) in those deaths negative for co-intoxicants.
Lammers et al. [84] emphasized the fact that there are no reported deaths in patients with narcolepsy-cataplexy, or indeed with other medical conditions, who are treated with the recommended doses of GHB. Moreover, it must be said that the reports of GHB usage in “date-rape” episodes, especially since the death of Kurt Cobain, a GHB abuser, has had an overblown negative press. Surely for each GHB-associated date-rape episode, there were a 100 or more rapes associated with amphetamines (especially methylamphetamine, Ecstasy), sedative-hypnotics (especially flunitrazepam, Rohypnol), esketamine (Ketamine), more alcohol, or other CNS active agents slipped into the drinks of the unfortunate victims. The excessive negative press coverage for GHB affected government officials, policy makers and the public to the point that it took years to have this useful medication available on prescription.
In summary, the objective evidence after worldwide experience involving several thousands of patients on GHB is that clearly GHB (sodium oxybate) , when taken at the recommended dosage levels, and in the absence of significant OSAS and respiratory depressants such as sedative-hypnotics, tranquilizers and alcohol, is one of the safest prescription drugs on the market. Moreover, in experimental animal studies, the LD-50 of GHB is some 20 times the therapeutic sleep-inducing dose. A UK parliamentary committee’s commissioned report found the use of GHB to be less dangerous than tobacco and alcohol in social harms, physical harm, and addiction [85].
Mechanisms of Action
What then is the mechanism, or rather what are the mechanisms, of GHB in delivering its clinical benefits? First, it must be said that a full description of the mechanisms of action and the neurochemical pathways of GHB is beyond the scope of this mainly clinical chapter. An excellent recent review is that of Xie et al. [86].
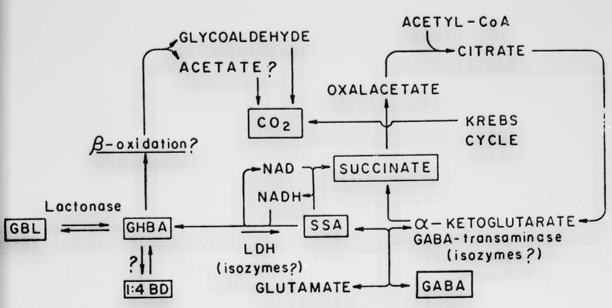
Given orally, GHB readily crosses the blood-brain barrier and is converted to a number of metabolites before entering the Krebs cycle and becoming CO2 and water. GHB is also endogenously produced in cells as a metabolite of GABA-B. There are many downstream metabolites of GHB, but the biological roles of these remain in general unknown. A summary of the main GHB pathways is presented in Fig. 63.6.