Fig. 1
Albumin levels in the basal ganglia at 24 h after a sham operation or endovascular perforation. Data are mean ± SD, n = 3–6 in sham, SAH with hydrocephalus (SAH HC), and SAH without hydrocephalus (SAH w/o HC), *p < 0.05 vs. the other groups
Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa (DARPP-32) levels were utilized to quantify basal ganglia neuronal injury at 8 days after surgery. We found that rats with hydrocephalus had more severe basal ganglia injury (DARPP-32/beta-actin: 0.38 ± 0.32 vs. 0.86 ± 0.45 in rats without hydrocephalus, p < 0.05; Fig. 2).
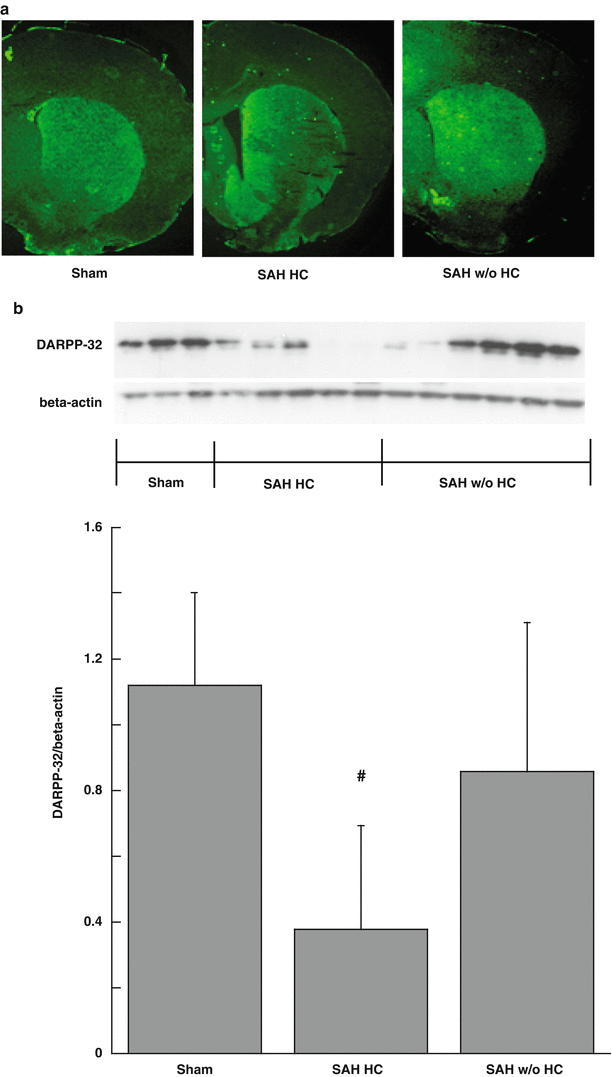

Fig. 2
DARPP-32 immunoreactivity (a) and protein levels (b) in the basal ganglia at day 8 after a sham operation or SAH. Data are mean ± SD, n = 3–6 SAH with hydrocephalus (SAH HC) and SAH without hydrocephalus (SAH w/o HC), #p < 0.05 vs. the other groups
Discussion
In the present study, we found SAH resulted in basal ganglia damage, which is associated with neurological deficits, hydrocephalus, and blood-brain barrier disruption after SAH. SAH-induced basal ganglia injury is not well studied, although basal ganglia hematoma has been reported in a traumatic SAH case [2].
We found that the incidence rate of basal ganglia (T2) lesions after endovascular perforation SAH in rats was 40 %. The size of the basal ganglia lesion correlated with the degree of behavioral deficit as assessed by the Garcia score. These results suggest that basal ganglia injury is common in the endovascular perforation SAH rat model and has an important role in SAH-induced neurological deficits. Attenuating basal ganglia damage may be an important target for improving functional outcome following SAH.
In rats that had a basal ganglia lesion after SAH, there was a marked increase (~4-fold) in the occurrence of hydrocephalus. In animals with SAH-induced hydrocephalus, there was also more neuronal death in the basal ganglia as shown by DARPP-32 levels. DARPP-32 is a reliable marker to quantify basal ganglia neuronal injury [5, 14]. The nature of the link between a basal ganglia lesion and hydrocephalus development is still unclear; for example, does a basal ganglia lesion induce hydrocephalus or vice versa, or are they independent events induced by another factor such as intraventricular blood? This needs further investigation. Our data did show that rats with hydrocephalus had greater blood-brain barrier disruption in the basal ganglia following SAH. It has been suggested that some cerebrospinal fluid absorption may occur across the blood-brain barrier [3], but this is controversial. Future studies need to determine the role of basal ganglia blood-brain barrier permeability in hydrocephalus development after SAH.
In conclusion, SAH caused severe basal ganglia damage in the rat. The occurrence of basal ganglia damage is associated with hydrocephalus development and blood-brain barrier disruption.
Acknowledgment
This study was supported by grants NS-073595, NS-079157, and NS-084049 from the National Institutes of Health (NIH), 973 Program-2014CB541600.
References
1.
Cahill J, Zhang JH (2009) Subarachnoid hemorrhage: is it time for a new direction? Stroke 40:S86–S87PubMedCentralCrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








