G×E (interaction) studies:
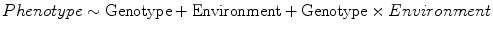
G×E Studies
In a sense, all genetics research can be viewed as G×E research because genetic effects are necessarily expressed in some environmental context (and vice versa). However, this is not a useful frame of reference because a complete understanding of the etiology of psychiatric phenotypes requires elucidation of causes that are ultimately genetic or environmental or interactive in nature. An important albeit underappreciated limitation of traditional heritability studies (i.e., twin studies) is that they are not able to estimate G×E effects. The potential consequences are serious because the existence of G×E will bias parameter estimates for genetic and environmental effects, as described in more detail below.
Regarding methodology, both molecular G×E and quantitative G×E studies are possible. The term “quantitative genetic” distinguishes studies of latent variables (e.g., A-C-E models using twins) from molecular genetic studies in which participants’ genotypes are measured. Quantitative genetic studies are also referred to as “behavioral genetic” studies, and we use the term “modified heritability” studies to make it clear to readers that such studies are (1) more similar to heritability (traditional twin) studies than they are to molecular genetic studies and (2) distinct from typical heritability studies, which cannot estimate G×E. In general, quantitative G×E studies are more suited to determining the relative magnitude of genetic, environmental, and G×E contributions to phenotypes, whereas molecular G×E studies can identify specific genetic and environmental risk factors. Finally, the study of G×Es has emerged out of both the genetic and environmental traditions of research.
The “Form” of G×Es
Visual depiction of G×Es, in line or bar graphs, helps to explain the concept of G×E. As demonstrated in Fig. 9.1, phenotype is always depicted on the y-axis. In this example, the phenotype is depression. Either the genetic variable or the environmental variable can be depicted on the x-axis. Here, the environmental variable (maltreatment) is depicted on the x-axis. Genotype is represented as lines G1, G2, and G3, corresponding to minor allele homozygote, heterozygote, and major allele homozygote genotypes. The existence of an interaction is indicated by the fact that the lines are not parallel. The presence of an environmental main effect is indicated by the fact that the trend lines for all genotypes have positive slopes (thus the overall effect of environment has a consistent direction). However, the slope of lines is different for each genotype, indicating that the effect of the environment depends on genotype, which is the definition of an interaction. It is also necessarily the case that the inverse is true: the effect of genotype depends on the environment. The form of an interaction—which can be observed in a graph—can be important conceptually and statistically, as discussed later—so we describe possible forms of interactions here. In addition to the G×E in Fig. 9.1, which is referred to as a “crossover interaction” because the lines in the graph cross, there are also “spreading,” “diathesis–stress,” “other,” and “pure crossover” forms of interactions, as depicted in cells A1, B1, C1, and D1, respectively, in Fig. 9.2. This terminology can be confusing, however, as illustrated by comparing the interactions in the left column with those in the right column. For each row in Fig. 9.2, a single interaction is depicted, but the form of the interaction can appear different depending on the arbitrary choice of which variable—genotype or environment—is placed on the x-axis. The form of interactions A1, B1, and C1 is different from their counterparts: A2, B2, and C2. In contrast, the form of a pure crossover interaction will be the same irrespective of which variable (genetic or environmental) is placed on the x-axis, as illustrated in D1/D2 (though the angle and location at which lines cross depends on the specific interaction). Figure 9.2 illustrates the fact that precise descriptions of interaction are necessary to accurately convey the nature of G×E effects. For example, it is critical to know if risk and protective environments reverse depending on genotype (e.g., A1/A2 and D1/D2) and if risk genotype depends on environment (e.g., D1/D2).
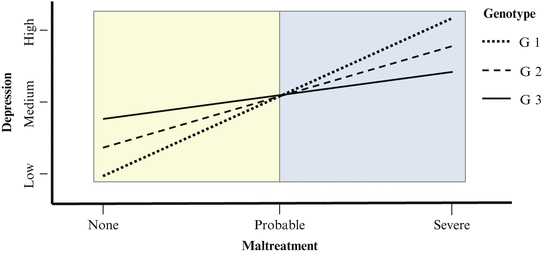
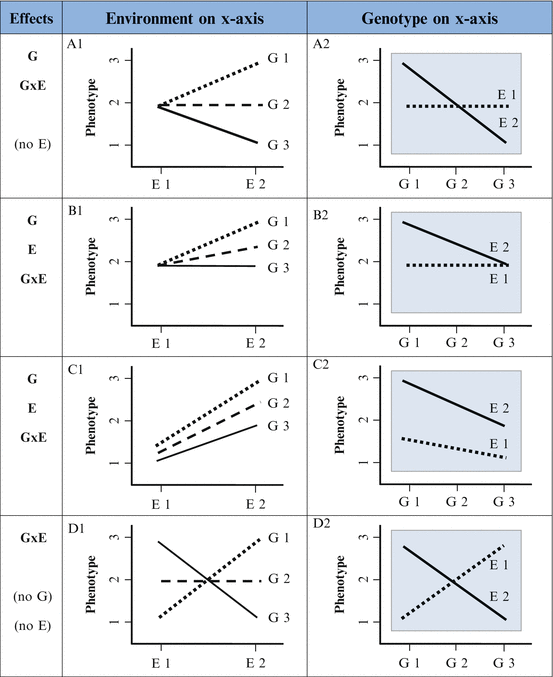

Fig. 9.1
Hypothetical example of gene–environment interaction (G × E)

Fig. 9.2
Form of interactions
Figure 9.2 also illustrates that not all interactions are created equal, so true replications of reported findings require interactions of the same form, as opposed to the mere presence of an interaction. This is the same as requiring that the direction of effect be constant in main effect studies, because results are certainly not replicated if effects are opposite to those originally reported. Though it is beyond the scope of this chapter, additional complexity exists regarding the form of interactions. Importantly, properties of scale used to quantify variables can also influence the form of interactions and have been extensively discussed (Aiken, West, & Reno, 1991; Eaves, 2006).
What G×E Is Not
As pointed out by Moffit et al. (2006), it important to clarify what G×E is not. It is not merely the combined effects of genetic and environmental effects. G×E is also distinct from gene–environment correlation (rGE), a phenomenon discussed later in this section. Finally, G×E is not epigenetics. Though the definition of epigenetics is evolving (Bird, 2007), there is widespread agreement that it pertains to DNA modifications that do not alter DNA sequence. Oftentimes these modifications (1) alter gene expression and (2) are brought about by environmental influences, and herein lies the confusion that has led some to conclude that epigenetics is a plausible “mechanism” for G×E (Bagot & Meaney, 2010; Meaney, 2010) when in fact it is much more closely aligned with environmental effects (Dick, 2011). The critical distinction between a phenomenon caused by both genes and the environment (and shared biological mechanisms underpinning the two) and G×E is that G×E implies variation in response to the environment due to variation in genotypes. Processes mistakenly described as G×E in the literature often fail to recognize this distinction. The bottom line is that G×E is not merely the combination of genetic and environmental effects but rather the existence of a dependency of genetic effect on the environment, and vice versa.
Historical Context for G×E Research in Psychiatry
G×E research is best understood via comparison with genetics research, which has been driven primarily by two factors: (1) genotyping technology and (2) theory. Before molecular genetic data (i.e., genotypes) were available, collective genetic effects were estimated in “heritability studies.” These were typically twin studies in which phenotypic correlations between monozygotic twins were compared to phenotypic correlations between dizygotic twins (Plomin, DeFries, & McClearn, 1990) (see Fig. 9.3) in order to yield estimates of genetic and environmental contributions to phenotypic variance. Heritability studies established that collective genetic influences are ubiquitous in behavioral and psychiatric phenotypes (Plomin et al., 1990).
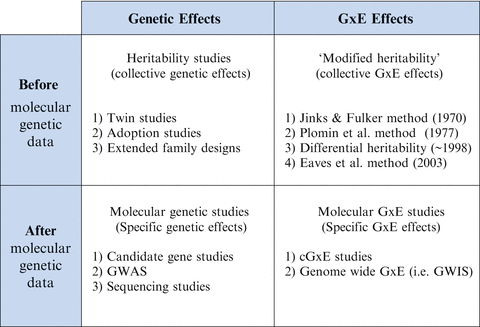

Fig. 9.3
Types of genetic and G × E studies, before and after the advent of molecular genetic data. GWAS genome-wide association study, GWIS genome-wide interaction study
Modified Heritability Studies
There was and still is a controversy about how important G×E effects are in psychopathology. For many years, heritability studies could not estimate effects of G×E. It was known that G×E effects could bias parameter estimates in heritability studies, but the magnitude of the bias was completely unknown. There was also confusion about how G×E effects would bias parameter estimates. Many believed that G×E effects could only contribute to the genetic (A) term, but G×E can also contribute to the non-shared environmental (E) term. The difference depends on which source of environmental variation interacts with genetic variation; if A and C interact, G×E effects appear as A; if A and E interact, G×E effects appear as E (Purcell, 2002). Thus, unrecognized G×E effects can masquerade as genetic and/or environmental effects.
Modified heritability studies shed some light on this issue, but leave many questions unanswered, as described in this section. Unlike traditional heritability methods (i.e., twin studies, mentioned above), methods for simultaneously estimating G×E, gene–environment correlation (rGE), and separate genetic and environmental effects were not available until the turn of the twenty-first century (Eaves, Silberg, & Erkanli, 2003). Thus, the full potential of these methods has still not been realized.
Four Types of Modified Heritability Studies
In 1970 Jinks and Fulker developed a method that indicated the presence of G×E as evidenced by a correlation between A and B for pairs of MZ twins:
(A)
The sums of scores for a phenotype of interest
(B)
The differences of scores for a phenotype of interest
Twins reared together and twins reared apart could be used, but twins reared apart were preferable because fewer assumptions were required (Jinks & Fulker, 1970). The second modified heritability method, developed by Plomin and colleagues in 1977, is usually thought of as a method involving twins reared apart, but can be used with any genetically related people as long as environments are uncorrelated (Plomin et al., 1977). Many groups including Heath, Eaves, and Martin (1998) used the third modified heritability method, which assesses the possibility of different heritability estimates across levels of a measured environmental variable (i.e., differential heritability across environment). It involves partitioning a sample into subgroups by some measured environmental variable and comparing heritability estimates across subgroups. For example, heritability of depression was found to vary by marital status and heritability of IQ by socioeconomic status (SES) (Heath et al., 1998; Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003). Finally, in 2003 Eaves and colleagues developed a fourth method that simultaneously estimates parameters for G, E, G×E, and rGE (Eaves et al., 2003). This is a tremendous advance because previously these parameters could only be estimated in independent methods and their biases on one another could not be determined.
Findings from Modified Heritability Studies
Firm conclusions from the four types of modified heritability studies are not available and the reasons for this situation are briefly described below. Jinks and Fulker’s method yielded few if any positive results for the presence of G×E interaction. However, this cannot be taken as strong evidence against the presence of interactions because power was likely low for this method given the modest sample sizes used at the time (e.g., 40 twin pairs, 29 twin pairs) (Jinks & Fulker, 1970). Additionally, if twins reared together are used for this method (as is typically the case due to the rarity of twins reared apart), purely environmental effects can be confounded with G×E effects (Plomin et al., 1977). Plomin et al.’s method also failed to generate positive results for interactions in the original report (Plomin et al., 1977), but the authors suggested that the method needed to be applied more widely. There appear to be no systematic reviews of results from this type of methodology, so it is unclear whether or not the existence of G×E interactions is supported by these studies.
The third modified heritability method of demonstrating G×E met with considerably more success in terms of initial findings consistent with G×E. For example, genetic contributions to depression (in women) appeared to be stronger for unmarried women than those in marriage-like relationships (Heath et al., 1998). Adolescent alcohol use was found to be more determined by genetic factors in urban environments as compared to rural environments (Dick, Rose, Viken, Kaprio, & Koskenvuo, 2001; Rose, Dick, Viken, & Kaprio, 2001), and the heritability of IQ was found to vary by SES (Turkheimer et al., 2003). Tsaung and colleagues provide an excellent review of these findings, and they draw the general conclusion that—in the presence of more permissive environments—genetic effects manifest themselves more strongly. In more restrictive environments, genetic effects are not expressed variably, and heritability estimates are lower (Tsuang, Bar, Stone, & Faraone, 2004).
While very intriguing, findings of differential heritability across measured environments need to be replicated and analyzed in meta-analyses before they can be considered to be robust. Regarding the example of differential heritability of IQ by SES, a recent large study failed to confirm the initial findings (Hanscombe et al., 2012). Moreover the replication history of this putative interaction has been mixed with some positive findings, some null findings, and even effects that go in the opposite direction of the original report (Hanscombe et al., 2012). Another limitation of this method is that rGE can cause spurious interactions—thus researchers must be careful to exclude environmental variables involved in rGE. This can be difficult because heritability studies often demonstrate genetic influences on environmental variables. Moreover, genetic influences often influence environmental variables thought to be causally related to psychiatric disorders (e.g., stressful life events).
The fourth modified heritability method, developed by Eaves and colleagues, can simultaneously estimate genetic, environmental (shared and non-shared effects), G×E, and rGE effects (Eaves et al., 2003). Though it is beyond the scope of this chapter to review all findings from this method, it is informative to describe the unique conclusions derived from its first implementation. Eaves and colleagues found that both latent G×E and rGE terms significantly improved model fit, implying that estimates of genetic and/or environmental effects in traditional twin designs would be biased. Specifically they found that—if a traditional twin design had been used—(unrecognized) G×E effects would have obscured a relatively large effect (17 %) of genes with specific effect on depression (not anxiety plus depression). This is important because a common finding in bivariate heritability studies is that there are not distinct genetic influences for anxiety and depression. Second, the influence of non-shared (E) environmental effects would have been grossly overestimated if the G×E term was not included in the model. Finally, the direct effect of life events on depression would have been overestimated, if rGE was not modeled (Eaves et al., 2003). In sum, initial results from this methodology suggest that G×E and rGE make sizeable contributions to depression, and failure to model them causes misleading conclusions about the etiology of depression. If confirmed, these results suggest that genes—acting via G×E and rGE—are even more important for depression than previously thought.
Summary of Results from Modified Heritability Studies
At least four major types of modified heritability studies have been developed for estimation of G×E effects (see Fig. 9.3). The first was developed in 1970 and the most recent in 2003 (Eaves et al., 2003; Jinks & Fulker, 1970). Little evidence consistent with G×E was found using the first two methods (Eaves et al., 2003). This may have been partially due to low power given the small sample sizes used at the time and the difficulty of finding twins reared apart, which is the ideal sample population for both methods. The third type of modified heritability study compares differences in heritability estimates for subgroups stratified by some measured environmental variable. Many positive findings have been reported using this method, but meta-analyses are needed to determine if results are true. Finally, the last modified heritability method is novel in that it allows for simultaneous estimation of G×E, rGE, and various genetic and environmental effects. Its initial use demonstrated evidence for both G×E and rGE. Importantly, it also demonstrated how failure to model G×E and rGE caused meaningful changes in interpretation of the findings. For example, some of the non-shared environmental influences were actually G×E influences. Overall, findings from modified heritability studies provide preliminary evidence that G×Es exist, but more research is needed to confirm that these types of G×E results can be consistently replicated.
Technological Advances Make Molecular Genetic Data Available
With the advent of molecular genetic data—meaning specific, measured genotypes—candidate gene studies dominated the genetics literature. Unlike heritability studies, candidate gene studies used unrelated individuals in case-control or population samples. Data were (or could have been) analyzed in a regression framework. Though these studies were first greeted with great excitement because they purported to identify the specific genetic variants responsible for collective genetic effects identified in heritability studies, they were soon challenged in psychiatry and in other medical fields. Candidate gene findings proved to be unreliable and the literature filled with null and inconsistent results (Colhoun, Mckeigue, & Smith, 2003; Ioannidis, 2005). Explanations offered for the puzzling phenomenon of non-replication were low power, population stratification, differences in measurement, and G×Es.
Emergence of Candidate G×E (cG×E) Studies
It was in this climate—widespread confusion regarding the failure of candidate gene results to replicate—that candidate G×E (cG×E) studies emerged. These studies were welcomed by many because they provided an answer to the troubling question of non-replication. The idea that environmental factors were important also resonated with environmental researchers, and many viewed Caspi’s work as a much-needed acknowledgment of the role of nongenetic factors in genetics research. Though the validity of specific results from cG×E studies was later challenged, excitement about these studies was evidenced by the publication of two cG×E studies in the journal Science (Caspi et al., 2002, 2003) and many others in leading journals.
The way in which G×Es could explain all types of inconsistent results can be seen by examining Fig. 9.1. Assume that a hypothetical candidate gene study sampled participants from the low range of environmental exposure (yellow). In this sample G3 would appear to be the risk allele. In contrast, if a second sample consisted of participants from the high range of the environmental exposure (blue), then G1 would appear to be the risk allele. Finally, if a third study sampled participants across the entire range of environmental exposure, then no genetic effect would be observed. Thus, G×Es can theoretically explain any pattern of inconsistent results. In other words, the population from which you draw your study can determine your results, if a G×E such as the one depicted in Fig. 9.1 exists.
Conceptual and Theoretical Issues Regarding G×E
Are Genetic, Environmental, and G×E Effects Separable?
Throughout the history of G×E research, the question of whether or not G×E effects are separable from genetic and environmental main effects has been asked on many occasions. The answer is yes (though it is not necessarily intuitive); G×E effects are meaningfully and actually separable from genetic and environmental effects. Plomin and colleagues explained this elegantly in 1977, making the point that “interactionism,” which they define as the idea that “environmental and genetic threads in the fabric of behavior are so tightly interwoven that they are indistinguishable,” is simply false at the population level. To be clear, it is true that—for an individual—genetic effects cannot be expressed in the absence of an environmental context just as environmental effects necessarily manifest themselves in the context of an organism’s genome. However, at a population level, it is possible to distinguish genetic from environmental effects. For example, if monozygotic twins differ, it is clear that those differences are due to environmental variables, because monozygotic twins share effectively 100 % of their genetic material. Thus, while it is important to keep in mind that genetic and environmental effects on behavior are both relevant—distinct genetic, environmental, G×E, and rGE effects exist, and a full understanding of the etiology of psychopathology requires precise delineations of these effects.
Gene–Environment Correlation (rGE)
Gene-environment correlation (rGE) means exactly that: a correlation between genetic and environmental variables. Typically cited mechanisms for the development of rGE invoke the effect of genotype (Plomin et al., 1977), but it is also possible that rGE could result from the environment, particularly over a longer time scale and through the course of evolution. For example, skin pigmentation (which is partially genetically determined) varies based on latitude (an environmental variable) thus inducing a correlation between certain environments and certain alleles in human populations.
Regarding classification, there are three commonly described types of rGE: “passive,” “reactive,” and “active” (Plomin et al., 1977). Plomin makes the point that these terms are not mutually exclusive and that any combination of rGE effects may occur in a given example. Passive rGE occurs when children are given certain genotypes, which are linked to certain environments. For example, athletic parents may provide both alleles and environments that are conducive to athletic performance (e.g., playing catch, encouraging participation in sports). Reactive rGE occurs when children’s genotypes bring about certain environments. For example, coaches may recognize children’s (genetically influenced) athletic abilities and provide situations conducive to further athletic development (e.g., more playing time, offers to be on competitive teams). Finally, active rGE involves children’s own seeking of environments, based on their genotypes. For example, athletic children may be more inclined to practice sports and may try out for sports teams more often. All of these examples involve genetic and environmental effects that act in the same direction (here, to increase athletic performance), but this is not a requirement for rGE. Rather, all that is necessary for rGE to exist is a systematic relationship between genetic and environmental variables, the effects of which can act in the same or opposite direction.
What Form(s) of G×E Exists in Nature?
A major question facing researchers pertains to the form of G×Es that affect psychiatric phenotypes. Are G×Es crossover in form? Are they non-crossover? Do all forms exist? Currently no one knows the answer to these questions, but there are both empirical and conceptual clues as to what we might expect. In plant species and nonhuman animals, interactions are well documented and common. Importantly, this includes crossover interactions, which are commonly found in experimental settings and nature if a wide distribution of environments is studied (Ceccarelli, 1996). Thus the argument that crossover interactions are “biologically implausible” is simply inconsistent with known biology. In the cG×E literature, all forms of interactions have been reported, including many crossover interactions. However (as described below) such interactions have yet to be conclusively validated in replication studies and meta-analyses, so observed evidence from the cG×E literature should not be treated as fact. Nevertheless, this empirical evidence from humans, animals, and plants suggests that we are likely to observe all forms of interactions impacting psychiatric phenotypes.
Conceptually, there are arguments in favor of both crossover and non-crossover interactions. Regarding non-crossover interactions, it stands to reason that these almost certainly exist if one considers “reaction to the environment” phenotypes. For example, anxiety in response to a stressor would be an example of a “reaction to the environment” phenotype as would sadness in response to the loss of a relationship. Both of these examples could conceivably contribute to risk of depression. If such “reaction to the environment” phenotypes are influenced by genes, then G×Es necessarily exist because this implies that genes influence one’s reaction to the environment. As genetic influences on behavior are ubiquitous, it stands to reason that “reaction to the environment” phenotypes would also be influenced by genes, and consequently we can infer the existence of non-crossover G×Es (e.g., some people will experience more sadness in response to the loss of a relationship than others and some people will be more anxious in response to stressors than others).
In contrast, some have questioned the likelihood of certain crossover interactions. They wonder if it could ever be the case that the loss of a relationship would decrease depression (in individuals with certain genotypes). Likewise, they question whether childhood maltreatment could ever be expected to bring about positive impacts on psychiatric health—such positive effects are a necessary consequence of pure crossover interactions. Extreme examples such as these seem to defy common sense and they may not exist. However, as some have proposed, there may be genetic differences in susceptibility to environmental influences (the so-called dandelion-orchid or differential susceptibility hypothesis) (Bakermans-Kranenburg & van IJzendoorn, 2006). The “differential susceptibility” hypothesis differentiates itself from the diathesis–stress hypothesis by stating that some individuals will be more susceptible to both positive and negative influences, whereas other individuals will be relatively impervious to the impacts of the environment. In contrast, the diathesis–stress hypothesis may be rigidly construed as only pertaining to negative influences.
Finally, evolutionary considerations are relevant for both the form and the magnitude of interactions. Simply put, any genotype that leads to a net negative impact on fitness (successful reproduction of an organism) will be removed from the gene pool over time by the forces of natural selection. Moreover, there is a well-established inverse relationship between the magnitude (effect size, essentially) of the negative effect of an allele and the length of time that it will remain in the gene pool: more deleterious alleles will be removed more quickly. Thus, the expectation is that alleles with negative effects will tend to have small effect sizes. Notably, this prediction is overwhelmingly consistent with what has been observed across all of human genetics: alleles for traits related to fitness tend to have tiny effect sizes (e.g., odds ratios <1.2). As it pertains to G×Es, evolutionary theory suggests that effect sizes for interactions involving a net negative effect of a particular allele (as in diathesis–stress interactions) will be tiny. However, this by no means precludes the possibility of diathesis–stress interactions (just as it does not preclude the existence of genetic effects); it merely suggests that effect sizes for individual diathesis–stress interactions will be tiny.
In contrast, pure crossover interactions offer the possibility of G×Es with large effect sizes with no net negative effect of any particular allele. Thus, large crossover G×Es are consistent with evolutionary theory and offer hope that single loci may make substantial contributions to psychiatric phenotypes. This exciting possibility is important and substantiates claims that the search for G×Es is important, but it should also be tempered by knowledge that this mechanism may be unlikely (Keller & Miller, 2006). In sum, the range and distribution of forms of G×Es impacting psychiatric phenotypes is not known, yet we can be relatively confident that large diathesis–stress G×Es will not be identified (because they would also have large genetic effects and evolutionary forces would remove such alleles from the gene pool). It remains to be seen whether crossover interactions with large effect sizes will be identified.
How Is the Environment Defined and Measured?
There is no single correct definition of the environment because it can be conceptualized in many ways. Bronfenbrenner’s ideas about the nested nature of environmental influences are useful in conceptualizing the environment from the most distant (e.g., culture, economic systems, history) to more proximal (e.g., extended family, neighborhoods), and even more proximal variables such as siblings, peers, and immediate family (Bronfenbrenner, 1979). In cG×E research, various stressors are most commonly investigated. In addition, treatments can be classified as environmental variables and can be either pharmacological or psychological (talk therapy) in nature. Taken to a more extreme point of view, biological states could also be considered to be environmental variables (from a gene’s perspective). For example, expression of a gene may differ in a male versus a female body. Likewise, the effect of a toxin or illness on the body may alter the expression of a gene. Each of these conceptualizations of environmental variables is valid (assuming no rGE), and potential disagreements about classification of a variable as environmental (or not environmental) can be easily managed if the operationalization of environmental variables is precise. Thus, what is most important in G×E research (with respect to defining the environment) is precise description of the environmental variable and how it was measured in the context of a particular study. As with any variable, higher-quality measurement is preferable to lower-quality measurement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






