Fig. 1
Diagram depicting the organization of circuits involved in pain facilitation from the DRt. This medullary area is involved in a feedback reciprocal loop with the spinal cord, which increases (+ symbol) pain facilitation. The DRt is also connected with several brain structures involved in pain modulation such as the rostroventromedial medulla (RVM), the locus ceruleus (LC), and the periaqueductal gray (PAG). Information from higher brain centers also reaches the DRt, such as the hypothalamus (Hyp), amygdala (Amy), insular (Ins), somatosensory (Som), and motor (Mot) cortices. Input from the anterior cingulate cortex (ACC) appears to reinforce descending facilitation from the DRt. The main source of noradrenergic fibers to the DRt derives from the LC
2 Materials
2.1 Vector Construction
HBS Solution
20 mM HEPES.
135 mM NaCl.
5 mM KCl.
5.5 mM glucose.
0.7 mM Na2HPO4.
Adjust pH to 7.05.
Store at room temperature (RT).
Culture Medium
1 L minimum essential medium Eagle (MEM; Sigma Aldrich).
43 mg gentamicin sulfate (25 mg/L).
995 ml H2O.
2.2 g NaHCO3.
Filter and store at 4 °C. Immediately before use, add cosmic calf serum to 10 % and l-glutamine 1×.
l-Glutamine 100×
2.92 g l-Glutamine.
99 ml H2O.
Filter and store at −20 °C.
PBS-D (10×)
137 mM NaCl.
2.7 mM KCl.
8.0 mM Na2HPO4.
1.5 mM KH2PO4.
Autoclave and store at RT.
TE Buffer
0.61 g Tris.
0.186 g EDTA, Na2·2H2O.
400 ml H2O.
Adjust pH to 8.0 with 1 M HCl and adjust volume to 500 ml.
Autoclave and store at RT.
2.2 Stereotaxic Injection and Immunodetection of Transduced Neurons
Buffer composition for immunoreactions:
Phosphate Buffer 0.1 M pH 7.2 (PB)
1,000 ml H2O.
13.79 g NaH2PO4.H2O.
17.4 g K2HPO4.
Store at 4 °C.
PBS
1,000 ml PB buffer.
9 g NaCl.
Store at 4 °C.
PBS-T
1,000 ml PBS.
3 ml Triton X-100.
Store at 4 °C.
Formol–thionin staining solutions:
Acid Acetone
4 vol acetone/1 vol acetic acid.
Prepare freshly before use.
Thionin
0.1 g thionin.
100 ml of 10 % formalin.
Filter and add three drops of acetic acid before use.
Store at room temperature (RT) for 6 months.
2.3 Effects of HSV-1-Mediated Gene Delivery
Solutions for microdialysis:
Artificial cerebrospinal fluid (aCSF)
150 mM NaCl.
31 mM KCl.
1.7 mM CaCl2.
0.9 mM MgCl2.
4.9 mM d-glucose.
Filter using a 0.2 μm filter and store at 4 °C for 2 weeks
Antioxidant solution
100 ml purified water.
700 μl perchloric acid.
400 μl 0.25 M EDTA.
2.6 mg sodium bisulfate.
Filter using a 0.2 μm filter and store at 4 °C for 6 months.
3 Methods
3.1 Vector Construction and Production
HSV-1 has a number of biological features that make it attractive as a gene delivery vehicle to the nervous system [1, 8, 9]. One important feature is its large genome (152 Kb) allowing many viral genes to be removed and replaced by large or multiple transgenes [10, 11]. HSV genes are expressed in a sequential order during a lytic replication cycle, with immediate early genes (IE) initiating the cascade of coordinated viral gene expression. The replication-deficient vectors used in our studies were generated from a vector backbone deleted for the essential IE gene ICP4 which blocks the expression of later genes in the gene expression cascade [12, 13].
We first engineered the DPZ vector (Fig. 2a) in order to express the Escherichia coli lacZ gene driven by the ubiquitous human cytomegalovirus (HCMV) promoter to study the transduction pattern of this vector in the brain after its injection into the DRt. Then we constructed another set of vectors (THz, THa, THTH) which already carry the HCMV promoter and the enhanced green fluorescent protein (EGFP) cDNA, inserted between UL36 and UL37 HSV genes (Fig. 2b). In this set of vectors, we inserted the rat tyrosine hydroxylase promoter (TH) in order to target transgene expression to noradrenergic DRt afferents. The transgenes placed under control of the TH promoter were (1) lacZ cDNA in the THz vector, (2) TH cDNA in the THTH vector, and (3) TH cDNA in antisense orientation in the THa vector (Fig. 2b) in order to decrease the expression of TH and consequently the biosynthesis of noradrenaline.
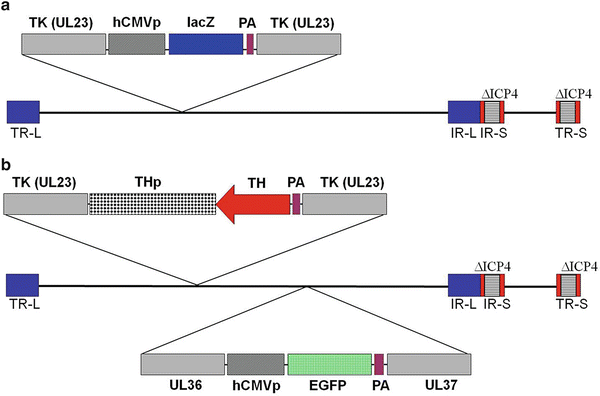

Fig. 2
Schematic representation of the HSV-1 constructs DPZ and THa. The vectors do not express the viral genes ICP4 (∆ICP4; deletion) and thymidine kinase (TK, UL23; insertional inactivation). (a) The DPZ vector carries a cassette, inserted into the TK gene, for the expression of the E. coli lacZ gene driven by the HCMV immediate early enhancer–promoter. (b) The THa vector carries a cassette inserted into the TK gene, containing the TH cDNA inserted in antisense orientation relative to the rat TH promoter, and a second cassette, inserted between the UL36 and UL37 viral genes, for the expression of the enhanced green fluorescent protein (EGFP). PA polyadenylation signal, TR terminal repeat, IR internal repeat, L long segment, S short segment
To construct the vectors, the first step consists of the cloning, by standard procedure, of each expression cassette (containing the promoter and the gene of interest followed by a polyadenylation signal) into a shuttle plasmid that contains HSV thymidine kinase (TK) flanking sequences. The second step consists of the digestion of (1) the ICP4-deleted viral DNA with the restriction enzyme PacI which cuts into the TK gene and (2) the shuttle plasmid with the restriction enzymes Ase I and Sal I generating a fragment of DNA containing the expression cassette flanked by the TK gene. The third step consists of the cotransfection of PacI-digested HSV DNA and the TK-flanked DNA fragment that will generate the recombinant vectors by homologous recombination (Sect. 3.1.1). After transfection, proceed with the titration of the recombinant virus (Sect. 3.1.2), then perform several rounds of limiting dilution to isolate and purify recombinants (Sect. 3.1.3), and finally prepare virus stocks with sucrose gradient purification to purify virus away from cellular and extracellular debris (Sect. 3.1.4).
3.1.1 Transfection for Generation of Recombinant Virus
The ICP4 gene is deleted from HSV-1 vectors; therefore, for replication in vitro, the missing essential gene must be provided in trans. For this, we use 7B complementing cell lines, which express ICP4 upon viral infection [11]. Standard tissue-culture practices are used to propagate and expand the 7B cell line in T-75 flasks. The procedure for 7B cells transfection is performed as follows:
1.
In a sterile tube, mix 5 μg of PacI-digested HSV DNA and 2 μg of TK-flanked DNA fragment containing the expression cassette.
2.
Add 300 μl of a solution containing 20 mM HEPES, 135 mM NaCl, 5 mM KCl, 5.5 mM glucose, and 0.7 mM Na2HPO4 (HBS, pH 7.05). Incubate for 20 min in ice.
3.
And finally add 20.5 μl of 2 M CaCl2, mix gently, and incubate at RT for 20 min.
4.
Wash 7B cells plated the day before in a single well of a 6-well plate at 75–80 % confluence with HBS.
5.
Add the transfection mixture to the cells and incubate for 40 min in the CO2 incubator.
6.
Add 2 ml of culture medium and incubate for 3 h in the CO2 incubator.
7.
Wash cells once with HBS.
8.
Add 1 ml of 20 % glycerol in HBS. Incubate at RT for 40 min, and then wash once with culture medium.
9.
Finally, add culture medium and incubate the cells for 2 days in the CO2 incubator.
10.
Harvest culture medium and cells using a cell scraper.
11.
Subject cells/medium to three cycles of freeze–thaw and sonicate for 10 s.
12.
Add 500 μl of 50 % glycerol in HBS and store at −80 °C.
3.1.2 Titration of the Recombinant Virus
1.
Prepare 2 tenfold dilutions (10−1 to 10−2) of the recombinant virus stock in 10 % glycerol with culture medium.
2.
Aspirate culture medium from 7B cells, at 80 % confluence, add 200 μl of culture medium with serum to each well (use a 6-well plate).
3.
Add 10 μl of each dilution (in duplicate) to each well. Rock the plate carefully to distribute the virus over the cell monolayer. Incubate for 1 h in the CO2 incubator.
4.
Aspirate the inoculum. Add 2.5 ml of 0.5 % methylcellulose in culture medium (to limit virus spread and produce readily visible plaques).
5.
Incubate until plaques are appropriate size (usually ∼2 days); aspirate the medium. Wash with 70 % ethanol for 5 min.
6.
Overlay and stain with 0.5 % crystal violet in 50 % ethanol for 50 min. Remove the stain, rinse with water, and air-dry.
7.
Count plaques and calculate the number of PFU per μl of the original stock.
3.1.3 Limiting Dilution for Virus Purification
1.
In a 15-ml conical centrifuge tube, add 30 PFU of the titrated original stock to 1 ml of 7B cells freshly trypsinized (from a T-75 flask and suspended in 5.5 ml of culture medium), 1 ml of culture medium, and 10 μl of 1 M HEPES buffer (pH 7.4). Place the tube on a Nutator rocker platform at 37 °C for 1 h.
2.
Add 8.5 ml of culture medium, mix, and pipette 100 μl into each well of a 96-well plate.
3.
Incubate for 2–4 days until most cells are rounded.
4.
Transfer the medium from all wells to a clean 96-well plate. Store this plate at −80 °C for use as a stock for the next round of limiting dilution.
5.
Process the original plate containing cells by dot blot, and perform blot hybridization to identify positives. For dot blot, use a probe against the gene of interest inserted in the recombinant virus.
6.
Thaw medium-containing plate from step 4. Transfer medium from each positive well to a screw-capped microfuge tube containing 400 μl of 10 % glycerol in culture medium.
8.
Carry out two additional rounds of limiting dilution using the stock of virus stored at −80 °C, as in steps 1–7 above.
9.
The virus stock from one positive well selected and stored at the end of the third round is used to expand the titer and extract viral DNA (see Note 1 ) in order to confirm the presence of the inserted cassette by PCR.
3.1.4 Virus Stock Preparation with Sucrose Gradient Purification
1.
Set up 3 T-225 flasks with 7B cells (6 ml of cell suspension resuspended in 60 ml of culture medium per flask).
2.
The next day, add 0.75 × 106 PFU of the recombinant virus in 11 ml of culture medium. Aspirate the culture medium of the 3 T-225 flasks (80–90 % confluent), and add 3.5 ml of recombinant virus solution. Incubate for 1 h in the CO2 incubator.
3.
Aspirate the viral inoculum. Add 70 ml of culture medium per flask and incubate for 48 h.
4.
Harvest cells. Transfer medium and cells to a sterile 250 ml centrifuge bottle, and centrifuge at 10,000 rpm with Sorvall SLA-1500 or GSA rotor for 10 min.
5.
Aspirate supernatant. Resuspend the cell pellets in 4 ml of cold culture medium. Transfer to a 15-ml centrifuge tube.
6.
Freeze–thaw three times, and after the final thaw, sonicate for ∼15 s on setting 3 of a cup-horn sonicator (Branson Sonifier 450). Add 9 ml of cold culture medium and centrifuge at full speed in a benchtop centrifuge for 10 min.
7.
Transfer supernatant to a 50-ml Sorvall tube containing 15 ml 30 % sucrose/PBS-D. Centrifuge at 18,000 rpm (38,000 × g) for 2 h at 4 °C in Sorvall SS-34 rotor.
8.
Carefully and thoroughly aspirate the supernatant. Resuspend the pellet in 10 % sucrose/PBS-D, using 450 μl per T-225 flask of cells. Pipette 100 μl aliquots and store at −80 °C.
3.2 Stereotaxic Injection of HSV-1 Vectors and Immunodetection of Transduced Neurons
3.2.1 Stereotaxic Injections
The HSV-1 vectors were stereotaxically injected in two DRt rostrocaudal parts of the left DRt following the coordinates of Paxinos and Watson [14] and using the interaural line as a reference to calculate the coordinates (Table 1). The procedure for HSV-1-vector injection is performed on Wistar rats (Charles River, Spain) weighing 285–315 g as follows:
Table 1




Stereotaxic coordinates used to target the left DRt
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








