Fig. 1
Dosing study showing transduction in coronal sections of healthy rat striatum following infusion of three different concentrations of AAV5-CAG-GDNF. Titer of the undiluted vector was 2 × 1013 GC/ml. The top four brains were injected with undiluted vector, showing massive unilateral expression of GDNF in the striatum and some in the cortex. The tenfold dilution showed a similar picture with good transduction of the striatum and cortex, however, to a lesser extent. Using the 100-fold dilution, however, transduction was poor. Numbers represent the animal identification number in the study
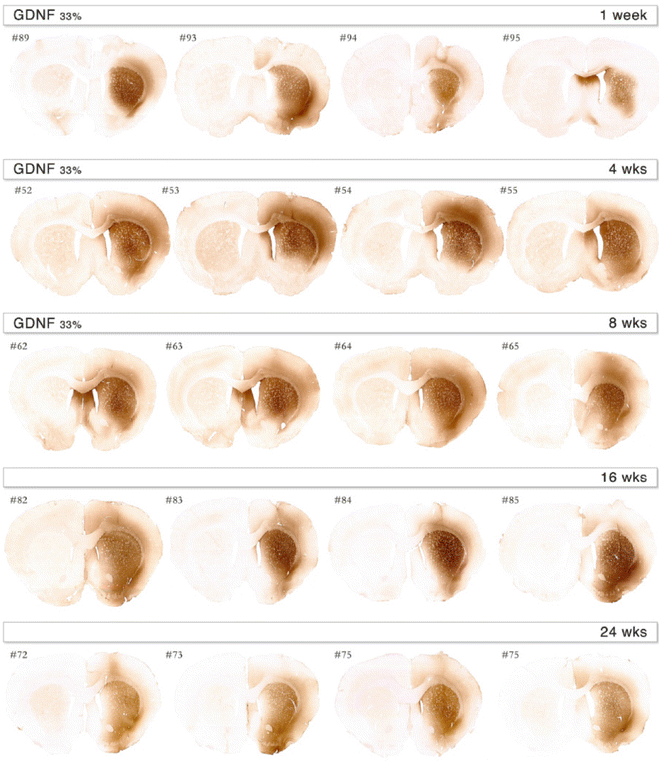
Fig. 2
Expression profile over time in the striatum following infusion of AAV5-CAG-GDNF showing transduction of rat brains. Based on the results shown in Fig. 1, we decided to dilute the vector to 33 % of its strength, resulting in 7 × 1012 GC/ml titer. Already at 1-week postinjection, transgene expression can be observed. The coverage of the expression is unilateral and mostly in the striatum with some expression in the cortex as well. Transgene expression is maintained over the period studied. At 4, 8, 16, and 24 weeks postinjection, good expression is observed in both before mentioned brain structures. Numbers represent the animal identification number in the study
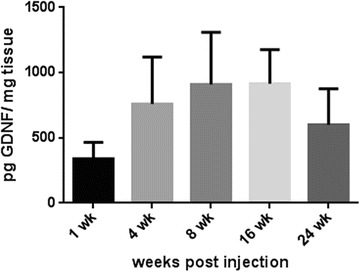
Fig. 3
Quantification of GDNF in the rat brain following infusion of AAV-GDNF over time. Titer of the vector was 7 × 1012 GC/ml. Values on the bars are mean ± SEM in pg GDNF. Expression of GDNF was measured from 1-week postinjection and was maintained throughout the study period. Range of levels was between 389 pg GDNF/mg tissue in the first week and 915 pg in the 16th week. These data suggest a long-lasting transduction of the transgene in the brain
Iba1 staining showed no massive activation. However, when compared to the non-injected side, a minor activation of microglial cells could be observed in the undiluted situation for both GFP and GDNF encoding vectors (Fig. 4). At the lower concentrations of vector, there was no obvious microglial response to the vector. Further analysis using ED-1 immunostaining indicates that GFP is more immunogenic than GDNF. Where the GFP vector mediated influx of macrophages at a tenfold dilution, the GDNF vector only mediated this in the undiluted form (Fig. 5).
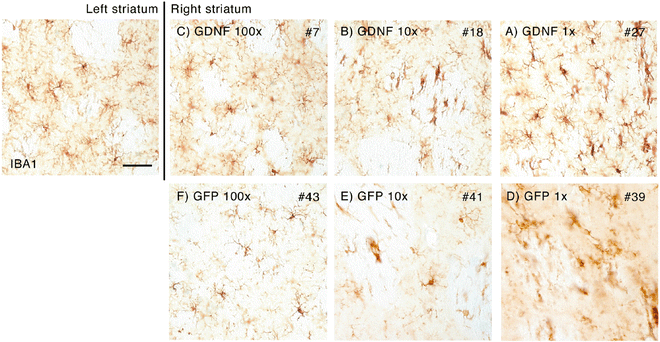
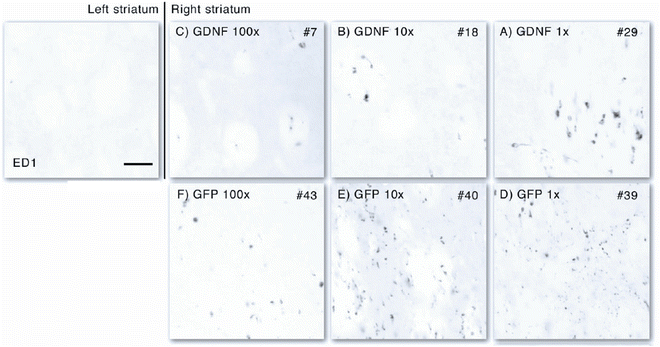

Fig. 4
Histological analysis of the immune response by immunostaining for ionized calcium-binding adaptor molecule 1 (Iba1). Iba1 is a microglia-/macrophage-specific calcium-binding protein and a good marker for neuroinflammatory responses. The left striatum is the non-infused striatum representing the healthy situation. Only some minor expression can be observed. In the 100- and 10-fold diluted vectors (both GFP and GDNF), Iba1 immunoreactivity is similar to that in normal tissue. However, in the highest concentration of vector, minor upregulation of Iba1 immunoreactivity can be observed suggesting microglial activation. Scale bar represents 0.05 mm

Fig. 5
Histological analysis of the immune response using ED-1, a marker for activated macrophages indicating neuroinflammatory responses. The left, non-infused striatum represents the healthy situation and shows no ED-1-positive cells. Only some minor expression of ED-1 can be observed in the 100- and 10-fold diluted GDNF vector (b and c). Only in the undiluted situation (a), some influx of macrophages can be observed. For GFP, the threshold is already at tenfold dilutions (d and e) and is minor at 100-fold dilution (f), indicating a more immunogenic profile of GFP compared to GDNF. Scale bar represents 0.05 mm
The biological effect was evaluated using histology with DA markers tyrosine hydroxylase (TH) and vesicular monoamine transporter (VMAT) in the 6-OHDA-lesioned brains of rats when AAV-GDNF was given prior to the lesion. The effect of the lesion is demonstrated using these stainings for DA markers (Fig. 6), and the protective effect of the AAV-GDNF is also clearly demonstrated. When quantifying the numbers of these VMAT-positive neurons, roughly a threefold decrease of neurons was detected in the PBS-treated group, whereas the GDNF-treated group showed an almost complete protection (Fig. 7). In the AAV-GDNF-treated animals, the substantia nigra appeared comparable to the uninjected side.
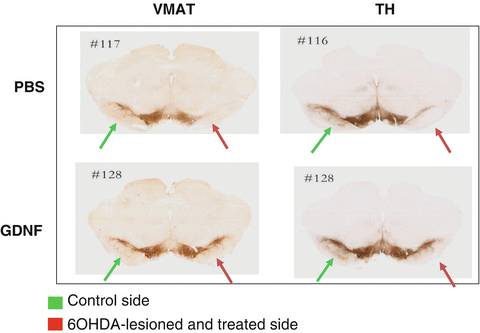
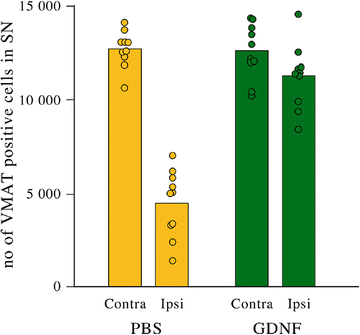

Fig. 6
Histological analysis of the representative micrographs showing substantia nigra stained for the dopamine cell markers vesicular monoamine transporter (VMAT) and tyrosine hydroxylase (TH), respectively. On the right side of the brain, a 6-OHDA lesion is performed (red arrow). The left side is the noninjured control side (green arrow). Please note the disappearance of VMAT- and TH-positive cells in the lateral part of the injured side in the PBS group (upper panel). Using AAV-GDNF as a therapeutic protective agent, neurons can be protected from the toxic insult

Fig. 7
Quantification of neurons in substantia nigra using VMAT as a marker for dopaminergic neurons showing protection against 6-OHDA lesioning. From each animal, three areas in the substantia nigra were chosen for quantification. Each dot in the graph represents a data point. In the PBS group, a clear decline of neurons is visible, whereas almost complete protection from 6-OHDA insult is observed in the AAV-GDNF group
These animals were also tested for forepaw usage in a cylinder test. The use of the lesioned limb is greatly impaired. Instead of 50 % usage in a healthy animal, roughly 20 % of the injured paw was used to stand on the cylinder wall for support. This was not ameliorated by the use of AAV-GDNF (Fig. 8).
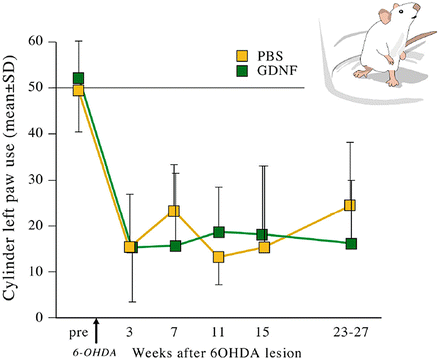

Fig. 8
Functional analysis using the cylinder test. In both PBS and GDNF groups, a decline in forepaw usage was observed at the lesioned side. Even though neurons in the substantia nigra were protected, there was no functional improvement using AAV-GDNF when compared to the saline-injected animals. This may be an indication that either the model is targeting a different population of neurons resulting in functional loss or that the expression of GDNF was not at the correct location as suggested before by Kirik and coworkers in 2002. Regrowing axons do not have a proper guidance trail when the trophic factor is not at the proper location
4 Discussion
Parkinson’s disease has many aspects that have to be targeted in order to obtain a clinical benefit. The focus should preferably be on preventing existing neurons from further degeneration as well as preserving the fibers and promoting regrowth. Regrowth of fibers innervating the correct target region is postulated to be possible using neurotrophic factors [22, 55, 57]. Reconnection and synapse formation need to take place. Finally, the brain needs to adapt to the newly formed circuitry. All these steps by themselves require complicated processes that could be part of the repair of affected brains. Therefore, protective therapy has to start as soon as possible before too many neurons have been eliminated, as reviewed in Fiandaca and coworkers [6].
In this chapter, we show that the AAV vectors encoding either GFP or GDNF are able to mediate gene expression in the striatum. The amount of GDNF present in the tissue was also quantified. Whether these amounts have a biological effect was tested in a rat model of 6-OHDA lesion. In this model, protection of neurons from toxin-induced death was demonstrated to be almost complete. It has to be noted that the AAV was given prior to the lesion. This is, however, an accepted model with a partial degeneration of DA neurons. However, based on the cylinder test, no functional improvement was observed. This could have several reasons. One of the reasons could be that the GDNF was not in the proper location as has been described by Kirik and coworkers [55]. In this study, they demonstrated that protection of nigral DA neurons against 6-OHDA-induced damage can be achieved by AAV-GDNF transduction of either substantia nigra or striatum, but that long-term functional recovery and regeneration of the lesioned nigrostriatal projection in the intrastriatal 6-OHDA lesion model is obtained only when GDNF is expressed over an extended period in the striatum alone. This suggests that paracrine rather than autocrine mechanisms are important for functional regeneration in the lesioned nigrostriatal DA system [55]. Moreover, the aberrant location may hinder new formation and/or regrowth of injured nerve fibers as suggested by Oudega and Hagg [57].
Another issue could be that the model has only limited value for predicting what could happen in a clinical setting. The 6-OHDA toxin may, as a side effect, damage more than just the neurons in the substantia nigra. This results in a more complex disease characteristic. As the current treatment is designed to have a local effect, this could be an explanation why no functional improvement is observed with the current behavioral test. These data show that AAV-GDNF is biologically active, supporting neuronal survival after 6-OHDA insult, and warrants further development. The next studies should include a more extensive dosing study, a larger animal study, and a biodistribution study, the latter being part of a toxicology and safety study as requested by the authorities.
Thus, in our opinion, the efficacy of the AAV5-GDNF in the rat model is unequivocal. These results are very encouraging and strongly support further development of this AAV platform for clinical application.
Acknowledgments
The authors are grateful to Anneli Josefsson, Ulrika Sparrhult-Björk, and Hongyan Liu for their technical support in this project.
References
1.
Website of the Parkinson’s disease foundation. http://www.pdf.org/en/parkinson_statistics and http://www.epda.eu.com/en/parkinsons/life-with-parkinsons/part-1/prevalence-of-parkinsons-disease
2.
3.
4.
5.
6.
Fiandaca MS, Bankiewicz KS, Federoff HJ (2012) Gene therapy for the treatment of Parkinson’s disease: the nature of the biologics expands the future indications. Pharmaceuticals (Basel) 5:553–590CrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








