The genetic counselor and neurologist meet with Mr. and Mrs. G. for the disclosure of the genetic testing results, which are positive for a known mutation in progranulin (GRN). Mr. G. is not surprised by this news, but is emotional over the confirmed risk to his children. The genetic counselor answers Mr. and Mrs. G.’s initial questions about the genetic test result. This leads into a discussion with the neurologist of its impact on his clinical diagnosis. A review of Mr. G.’s clinical evaluation is consistent with FTD. The genetic counselor contracts with them to follow up by phone, and to have an in-person meeting in one month.
By professional definition, genetic counselors help people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease. Genetic counseling is often a process, not a single session, involving interpretation of family and medical histories, education about inheritance, testing, management, prevention, resources, and research, and counseling to promote informed choices and adaptation to the risk or condition [1]. Given how rapidly genetics has entered the medical landscape, it is not surprising that genetic counselors are increasingly in demand for many specialty clinics.
Like many health professionals, genetic counselors can serve different roles, depending on the particular needs of a case. In the case of Mr. G., the genetic counselor’s initial role focuses on the collection and interpretation of the family history. Eliciting detailed family history information is a cornerstone of the genetic counseling process. In many cases, the dialogue surrounding the family history is the first interaction between the genetic counselor and patient, not only setting the stage for the next steps but also establishing a connection between the two parties. A detailed family history is an important diagnostic tool and one of the key issues in providing genetic counseling in the FTD setting [2, 3]. The collection of family history should not only focus on classic dementia symptoms, but also psychiatric histories and symptoms of movement disorders such as parkinsonism and motor neuron disease. A particular challenge in the collection of an FTD-specific pedigree is that many diagnostic terms currently used may not have been used in previous generations. Therefore, care is taken to note as many details as known about the age of onset and clinical symptoms from initial onset onward, or even to elicit family stories concerning a relative with possible neurodegenerative disease. Due to the behavioral and personality changes associated with FTD, family histories of psychiatric symptoms are not uncommon. Family histories that include the onset of psychiatric diagnoses later in adulthood should be considered with the same attention given to more classic reports of neurodegenerative disease. The resulting detailed pedigree, typically encompassing three or more generations, holds vital information that can be used to guide genetic testing decisions for the patient and family.
The following points can help detect reduced penetrance, which can hide or minimize a classic autosomal dominant pattern of inheritance:
Collect first- and second-degree relatives (children, siblings, parents, aunts/uncles, and grandparents)
Document current ages and ages at death
Do not assume a relative with late-onset neurodegenerative disease does not contribute to a possible genetic pattern
Misdiagnosis of FTD is common, especially in previous generations. The following points directly address common misdiagnosis concerns:
For relatives with neurodegenerative disease, document as many clinical details as possible, such as age and symptoms at onset, symptom progression, and source of diagnosis (e.g., family report, primary care physician, specialist, autopsy)
For relatives with movement disorders, ask about co-occurrence of cognitive decline (e.g., Parkinson’s disease with dementia) and vice versa (e.g., Alzheimer’s disease with parkinsonism)
Ask specifically if any relatives had diagnoses of mental illness, nervous breakdowns, or other psychiatric concerns during adulthood. Document details such as ages of onset, symptoms, successful or unsuccessful treatments, or even just family stories about the individual’s symptoms and behavior
For histories of substance abuse, document any details known about other difficulties, either physical or psychiatric, as well as any successful treatment or recovery periods
The discussion of genetic contribution to disease can bring a complicated combination of science and emotions. While genetics is often referenced in the news and popular literature, many patients do not have a high level of genetics education. Trying to absorb new genetic information, as well as information about FTD itself, can be overwhelming. Genetic counseling provides genetics education in more patient-friendly terms. Genetic counseling can also address the preconceived beliefs learned from friends or family members or through self-education most often via the Internet. However, with the understanding of genetics and inheritance, emotional responses such as sadness, fear, and guilt can arise. Genetic counseling should support and guide patients, as well as the patient’s family since genetic information rarely impacts the patient in isolation. As illustrated in many of the case examples, a genetic diagnosis in a patient will often lead to additional family members contacting the genetic counselor with questions and concerns of their own. In some cases, the genetic counselor may be able to take on these additional individuals as patients in their own right, providing them with the same level of personalized attention and care as their family member has received. Genetic counselors often serve as guides to the patient and family in their search of additional resources (e.g., support groups, psychiatric referrals) and information (such as clarifying which Internet sites contains patient-appropriate and scientifically correct information).
While not every FTD patient will need genetic counseling, the need to address genetics in the FTD setting has greatly increased over the past decade, with 15–30% of FTD cases being attributed to a known genetic cause and approximately 40% of patients describing a positive family history for neurodegenerative disease [4–7]. Thoroughly addressing genetic issues can often take more time than a physician’s clinical schedule allows, and can raise family issues that go beyond patient care. A referral to genetic counseling can provide the patient with access to a health professional whose specific goal is to focus on their genetic issues and questions, freeing the physician to spend more time on appropriate care and management of the patient. In the case of Mr. G., it was the combination of skills and evaluations by the neurologist and genetic counselor together that defined the need to discuss FTD genetics with the patient and provided Mr. G. and his family with a high level of care, education, and support. As research continues to uncover genetic causes and risk factors for FTD and other neurodegenerative diseases, it will be important for clinicians to have established relationships with genetic counselors in their geographic area [8] (www.nsgc.org).
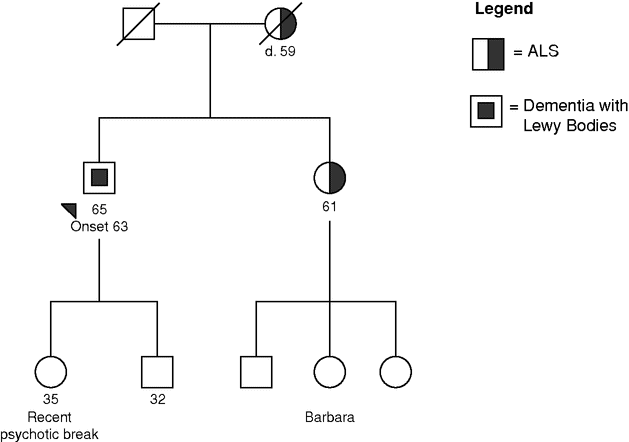
Mr. B. is a 65-year-old man with a two-year history of memory complaints and unsteadiness. He had recently retired from his job as a college professor because of trouble organizing his lectures. Additionally, his wife complained that when watching TV, he felt that the TV characters were in the room. Mrs. B. began to worry that he was depressed or psychotic, and shared her feelings with the primary care physician. After an examination, Mr. B. was referred to a neurologist. A neurologic and neuropsychological evaluation concluded that Mr. B. met criteria for dementia, with deficits in memory, visuospatial, and executive function. Additionally he was found to have mild rigidity, ataxia, and dysarthria. An MRI showed global atrophy with mild cerebellar and thalamic atrophy. Because Mr. B.’s sister had recently been diagnosed with ALS, the neurologist ordered an EMG, which was normal. Because of the memory deficits, psychological symptoms, blunted affect, and rigidity, the diagnosis of dementia with Lewy bodies (DLB) was given. Over the next six months, Mr. B. began exhibiting more agitation and paranoia. His speech became extremely perseverative, and he insisted on a strict schedule.
Mr. B.’s niece, Barbara, is newly married and wants to get pregnant. She is concerned about her family history of ALS. Not only had her mother been recently diagnosed at age 61, but also her maternal grandmother died of ALS at age 59. Barbara locates a genetic counselor specializing in neurogenetics by consulting http://www.nsgc.org, and makes an appointment to discuss her options. Barbara and her husband, Rich, come to the appointment. The counselor takes an extensive family history, noting the two cases of ALS. She asks if anyone else in the family has had any neurologic or psychiatric condition. Barbara responds that her uncle has some kind of dementia and something to do with PD. She also mentions that her 35-year-old first cousin, daughter of this uncle, has recently been hospitalized for a psychotic break. This cousin had always been normal until this nervous breakdown. Barbara thinks that her father’s illness might have precipitated her condition. Barbara’s father is living and well, as are her older brother and younger sister. Barbara’s uncle has a healthy 32-year-old son in addition to his daughter.
The genetic counselor discusses the genetics of ALS and says that two generations of first-degree relatives with the disease is concerning. She also mentions that the uncle’s condition could possibly be related. Barbara asks if she could be tested for the ALS genes. The genetic counselor explains that an affected family member would need to be tested first in order to make Barbara’s test meaningful. She explains that hereditary ALS could be caused by mutations in many genes, and not all genes are known. If a mutation is found in an affected person, then predictive testing could be performed for that mutation. Without knowing the family mutation, a negative result on predictive testing would be meaningless because a mutation could still exist in some unknown or untested gene. The counselor also discusses autosomal dominant inheritance and how finding a mutation in Barbara’s mother would put her at 50% risk for inheriting that mutation. If she harbors the mutation, she would most likely develop the disease and she would have a 50% risk of passing it to her children. However, discovering the gene in the family would also give the couple information needed for reproductive options. She explains that Barbara could elect to have predictive testing. If she has the mutation, the couple could decide not to have children, proceed with a pregnancy without testing, adopt, have an egg donor, have prenatal testing, or have pre-implantation genetic diagnosis (PGD). Through in vitro fertilization followed by PGD, embryos would be tested, and only those embryos lacking the family mutation would be implanted. She also says that it would be possible to do non-disclosing PGD should Barbara want to prevent transmission of the gene to offspring while not knowing her own genetic status.
The counselor points out that although Barbara can learn her own status if she wishes, if her result comes back positive for the mutation, she could not be told exactly when or what symptoms would develop. She might develop symptoms at an earlier or later age than her mother or uncle. Additionally, if she wants predictive testing, she should first obtain any insurance that she would want for the future. Although the federal Genetic Information Non-discrimination Act (GINA) protects against discrimination based on a hereditary family history for health insurance and employment, it does not cover long-term care insurance, life insurance, or disability insurance. If positive, Barbara could have a hard time obtaining these. Barbara said that she would talk with her parents and siblings. She is not sure that her brother would want to know about the possibility of a hereditary condition as he already has two children.
Barbara calls the counselor two weeks later to report that her parents have agreed to testing. Her sister agrees that the information is important. Her brother said that he would not want to test himself should a mutation be found, but would not deprive his sisters of their right to know. The family arranges with their neurologist to have a family counseling session followed by a blood draw. Because the family history includes both ALS and dementia, genetic testing for C9orf72 is ordered first with reflex testing to the rest of the ALS genetic panel. Results demonstrate a hexanucleotide repeat expansion in the C9orf72 gene in Barbara’s mother. At a meeting with both the neurologist and genetic counselor, this result is given to the patient, her husband, and their two daughters. The genetic counselor explains the meaning of this expansion and what is known and not known about its effect. She recounts how the gene displays autosomal dominant inheritance with high penetrance, but that the exact penetrance is not yet known. She also explains that the gene expansion could result in various phenotypes. She mentions that although behavioral variant FTD is the most common presentation of C9orf72-associated FTD, memory complaints and psychotic symptoms are not unusual. When Barbara asks if this information should be given to her aunt, the genetic counselor says she thinks it would be a good idea and offers to speak with her if she wishes.
Barbara schedules an appointment for predictive testing counseling. She understands that the protocol for predictive testing follows the Huntington’s disease (HD) protocol. She would have another counseling session with her husband to consider the impact of testing on each of them, Barbara’s family, and their future children. She would then undergo a neurologic examination to rule out early symptoms and a psychological evaluation to assess her mental status and ability to cope with results. Following testing, she would return for in-person results with her husband. If positive, they would be referred to an infertility center familiar with PGD.
Later that week, the genetic counselor receives a call from Barbara’s aunt, Mrs. B., requesting an appointment. Mrs. B. and the genetic counselor discuss the possibility of C9orf 72 testing for Mr. B. Mrs. B.’s greatest concern is keeping the information from her psychotic daughter. She is in recovery and Mrs. B. is terrified that the information could make her worse. Mrs. B. doubts that her daughter will ever have children and, therefore, the information would not be helpful. She refuses to consider that her daughter’s symptoms might be related to the gene. The counselor asks about her son. She says that her son might be interested, but that if he knew, it would be hard to keep the information from his sister. The counselor acknowledges that family secrets are difficult and suggests that she speak to her son and plan together.
Mrs. B. calls back to say the neurologist is arranging testing. The test reveals that Mr. B. has the C9orf72 expansion. The counselor offers to meet with Mrs. B.’s son any time in the future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree