Chapter 5 Head Trauma
Imaging of head trauma has a primary role both in diagnosing the extent of the traumatic injury and in expediently determining the appropriate therapy. The most efficient method of triage for acute trauma remains computed tomography (CT). It is fast and usually very accurate at detecting acute hemorrhage (high density on unenhanced scan) (Fig. 5-1). CT is excellent for assessing facial and skull fractures. Neurosurgeons are interested in knowing the precise source of the patient’s clinical problems with respect to the trauma. Their most overriding concern is whether there is a treatable lesion. Such lesions could be an epidural hematoma, a large subdural hematoma, actively bleeding parenchymal hemorrhage, brain herniation, or a significantly depressed skull fracture. CT does have some pitfalls of which the radiologist should be aware, particularly during the 4-hour board examination. Not all hemorrhage is high density. Isodense to low density hemorrhages are seen in patients who are severely anemic or in those with disseminated intravascular coagulopathy (DIC) or in the hyperacute actively bleeding stage (Fig. 5-2). Fluid-fluid levels and sedimentation imply active bleeding, which may be prolonged in patients on anticoagulants. CT does not easily detect extracerebral intracranial hemorrhages of the infratemporal region, subfrontal region, or posterior fossa. It also is less sensitive than magnetic resonance (MR) imaging in detecting diffuse axonal injury and vascular injury.
When trauma is evaluated with CT, review the images with brain, subdural, and bone settings at the workstation or demand films with those windows. The wide window setting aids in separating high-density blood from the high density of bone and is particularly useful in acute subdural and epidural hematomas, which can be thin and difficult to differentiate from the calvarium. Bone windowing is essential in the search for fractures, as is the combination of coronal and axial images with very thin sections, particularly of the skull base, temporal bone, orbit, and face (Fig. 5-3).
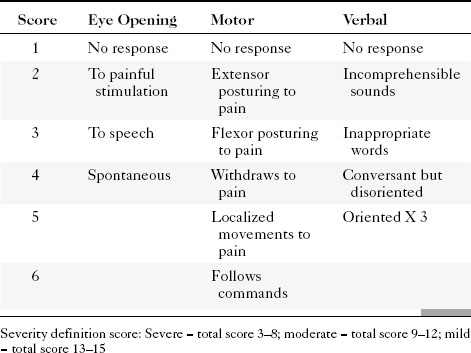
Patients with TBI have been classified into mild, moderate, and severe grades by the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine based on (1) a period of loss of consciousness, (2) loss of memory for events immediately before or after the trauma, (3) change in mental status, and (4) focal neurologic deficits. The Glasgow Coma Scale (GCS) was devised to provide a uniform approach to the clinical assessment of patients with acute head trauma and is the most frequently used means of grading the severity of injury within 48 hours (Table 5-1).
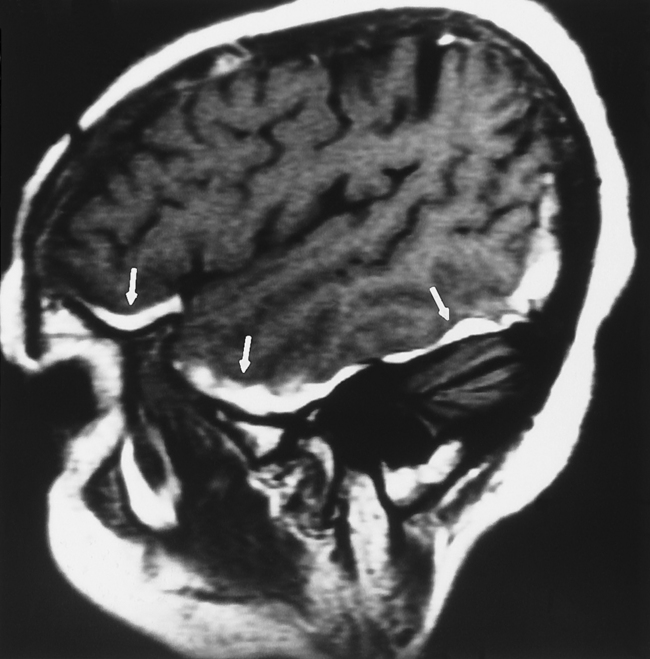
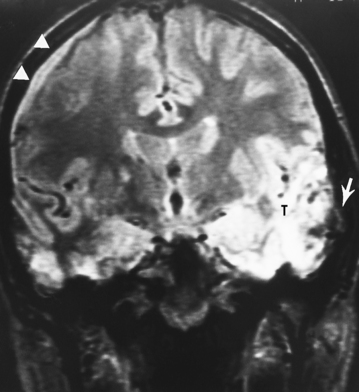
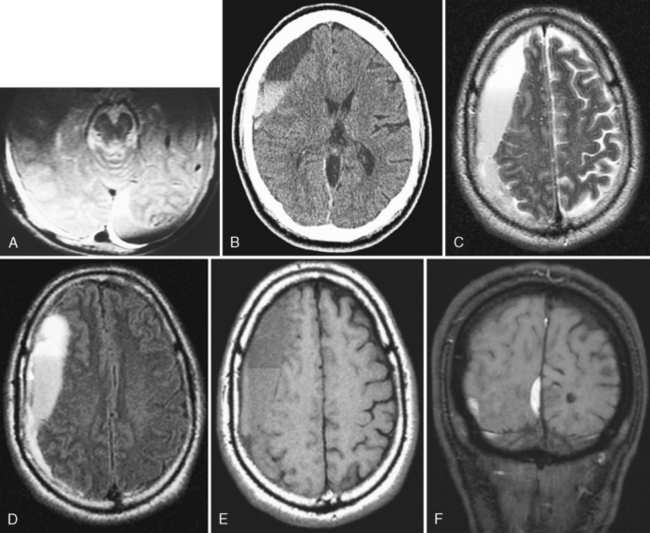
MRI is more sensitive than CT in detecting all the different stages of hemorrhage (i.e., acute, subacute, and chronic—although, within the first 4 hours, for hyperacute hemorrhage, CT may be more sensitive than low field MR). However, detecting acute subarachnoid hemorrhage is difficult if fluid-attenuated inversion recovery (FLAIR) imaging is not performed with the MR examination (Fig. 5-4). The direct multiplanar capability and absence of beam hardening artifact of MR enable excellent visualization of the inferior frontal and temporal lobes and the posterior fossa (Fig. 5-5). MR is more sensitive than CT in demonstrating DAI (see discussion of DAI under Primary Injury in this chapter), isodense subdural hematomas, minor deposits of hemorrhage with susceptibility-weighted scanning (particularly at higher field), and coexistent ischemia. CT/CT angiography (CTA) and MR/MR angiography (MRA) are both excellent means for detecting vascular dissection, but give the nod to CT for in–emergency department (ED) convenience.
EPIDURAL HEMATOMA
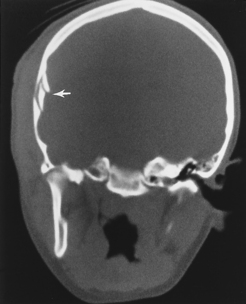
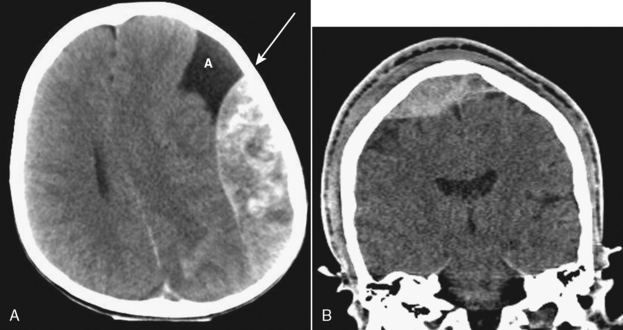
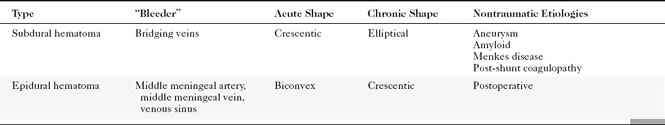
The potential space between the inner table of the skull and the dura is the epidural space. The most common cause of epidural hematoma is head trauma with skull fracture of the temporal bone (90% of cases) crossing the vascular territory of the middle meningeal artery or vein. In children, the greater elasticity of the skull permits meningeal vascular injury without coexistent fracture. Tears of the middle meningeal artery (60% to 90%) or venous structures (middle meningeal vein, venous sinus, or diploic veins; 10% to 40%) result in the extravasation of blood and acute epidural hematoma. After injury there may be a lucid interval (in 50% of patients) as the lesion expands before causing midline shift and deterioration (as opposed to DAI, where coma occurs immediately after the injury). Slower bleeding from the meningeal vein or dural sinus temporally delays symptoms. Chronic epidural hematoma has also been observed. Epidural hematomas are usually biconvex and are the result of the firm adherence of the dura to the inner table and its attachment to the sutures (Fig. 5-6). Most frequently, these hematomas are observed in the temporal parietal region. When fractures run through the middle meningeal artery and vein, occasionally a fistula may develop between the two. Pseudoaneurysm of the meningeal artery may also occur. Epidural hematomas of the posterior fossa result from tearing of a venous sinus and may be continuous with the supratentorial and infratentorial space. Venous epidural hematoma is most often noted in the pediatric population and carries a lower incidence of skull fracture.
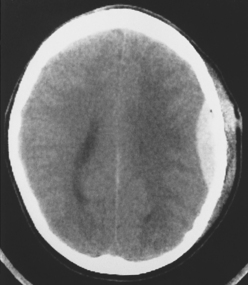
Figure 5-6 Computed tomography of acute epidural hematoma. Note the convex shape of the epidural hemorrhage.
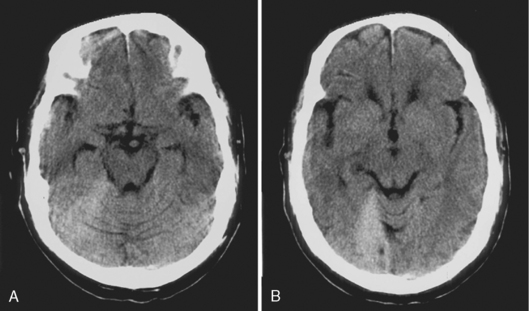
Computed tomography reveals a high density extra-axial mass acutely (Fig. 5-7). There may be some low density in the acute hemorrhagic mass, probably representing serum extruded from the clot. In these cases, perform bone window settings to examine the scout image for fractures and upper cervical spine subluxations not visible on axial sections. Chronic epidural hematomas reveal low density and peripheral enhancement, and they may be concave. Chronic epidural hematomas must be distinguished from other epidural masses such as infection, inflammation, and tumor, and from subdural lesions such as empyemas.
On MR, the extra-axial hemorrhage has different appearances depending on the interval between the traumatic event and imaging. If imaged less than 12 hours after trauma, acute hemorrhage demonstrates low intensity on T2-weighted images (T2WI) and isointensity on T1-weighted images (T1WI), whereas if the hemorrhage is imaged a few days after the incident (subacute hematoma), it is high intensity on T1WI (see Fig. 5-5). Intensity is not usually an issue because the biconvex morphology of the extra-axial mass in the setting of acute trauma makes the diagnosis obvious. One may visualize the medial dural margin as a hypointense rim on all pulse sequences. Linear fractures are poorly visualized on MR, but cortical veins and arteries are displaced inward as in any extra-axial lesion.
SUBDURAL HEMATOMA
Hemorrhage into the potential space between the pia-arachnoid and the dura is termed a subdural hematoma. Acute subdural hematomas result from significant head injury and are caused by the shearing of bridging veins (Fig. 5-8). This occurs because of rotational movement of the brain with respect to fixation of these veins at the adjacent venous sinus or dura. The subdural portion of the vein is not ensheathed with arachnoid trabeculae as is the subarachnoid portion and therefore is the weakest segment of the vein. Acute subdural hematomas also occur in the setting of the burst lobe (Fig. 5-9). Intracerebral clot is in direct continuity with the subdural hematoma. Penetrating injury can also result in acute subdural hematoma.
Subdural hematomas in the elderly, at discovery, are larger because of the generalized loss of brain parenchyma allowing greater unimpeded growth before mass effect and symptoms. Subdural and epidural hematomas are contrasted in Table 5-2 (also compare Fig. 5-6 with Fig. 5-8).
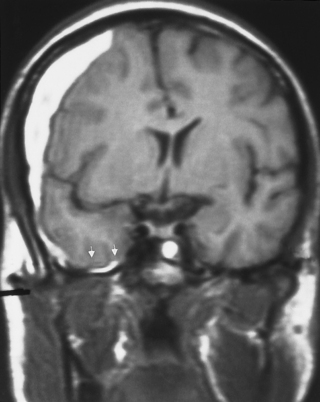
The temporal nomenclature of the subdural hematoma is arbitrary. Lesions occurring at the time of the initial injury are considered acute, although symptoms may take up to a few days to become manifest. Those lesions becoming symptomatic between approximately 3 days to 3 weeks are subacute (Fig. 5-10), whereas those lesions that are diagnosed after 3 weeks are considered chronic. Subdural hematomas generally arise over the convexities but can also occur in the posterior fossa, middle cranial fossa, or along the tentorium (Fig. 5-11).
Imaging Characteristics of Subdural Hematomas
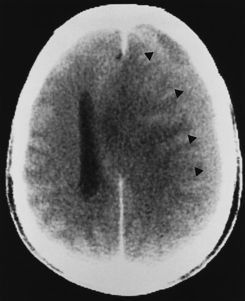
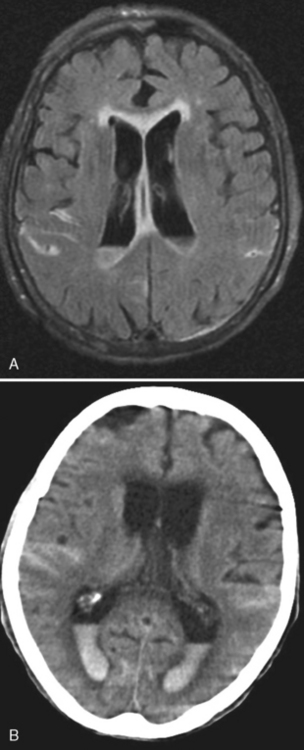
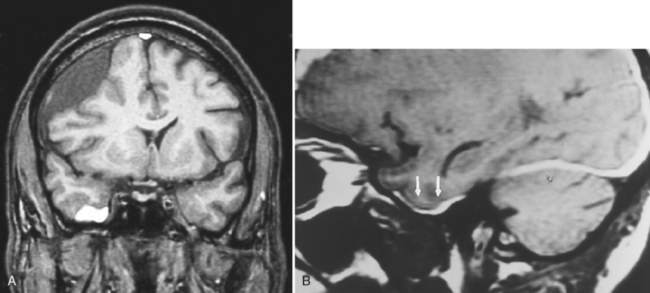
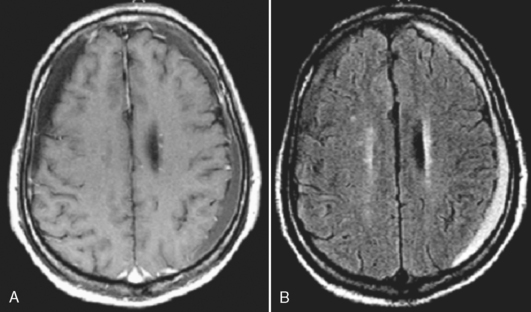
Subacute subdural hematomas, on the other hand, are usually isodense to low density; however, mass effect is common in significant subacute hematomas. The lesion usually is concave but occasionally may be convex when chronic, with inward displacement of the convexity veins, which course along the inner surface of the arachnoid. Although this shape may simulate an epidural hematoma, subdural hematomas are not confined by the cranial sutures and the chronic collections are low density. Intermediate window settings on CT are useful in separating thin subdural hematomas from the bony calvarium. This is obviously not an issue with MR. Subacute and chronic subdural lesions enhance (but who gives contrast in this setting?). This is caused by the vascularization of the subdural membranes (usually being thick outside and thin inside), formed between 1 to 3 weeks after injury, which enhance. These vessels are not associated with tight junctions. They leak contrast and may easily be torn, resulting in various blood products and growth of the subdural hematoma. Repeated episodic bleeding results in fibrous septations and compartments within the hematoma. Subacute and chronic subdural hematomas may also display layering with dependent cells and cellular debris and an acellular supernatant (Fig. 5-12). The dependent portion is of higher density than the supernatant. Hematomas may also occur between the leaves of the tentorium. Tentorial subdural hematomas fade out laterally and stop abruptly at the medial margin of the tentorium (Fig. 5-13).
Chronic subdural hematomas are usually low density (Fig. 5-14). High and low density levels observed in these lesions may be caused by rebleeding into the chronic subdural collection. There may be no history or an insignificant history of head trauma. Other contributing conditions include coagulopathy, alcoholism, increased age, epilepsy, and surgery for ventricular shunts. More than 75% of chronic subdural hematomas occur in patients over age 50 years. Chronic subdural hematomas occur in infants as a result of birth injury, vitamin K deficiency, coagulopathy, or child abuse. In fact, the presence of multiple-aged subdural hematomas should raise the spectre of nonaccidental trauma (see discussion of child abuse in this chapter). Rarely, chronic subdural hematomas may ossify or contain fat.
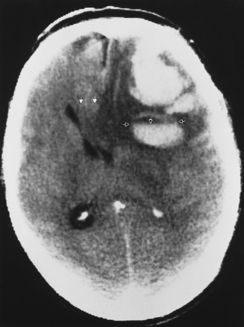
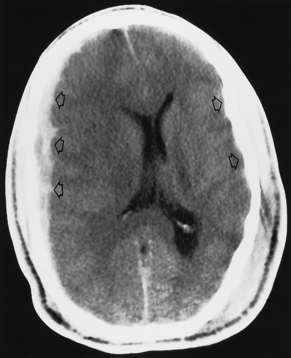
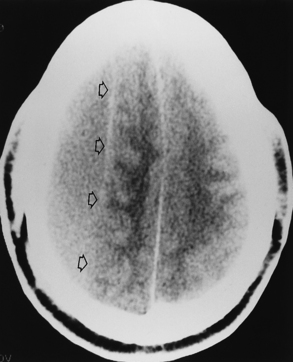
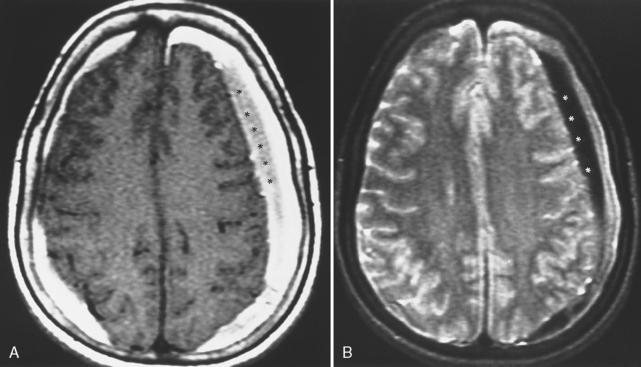
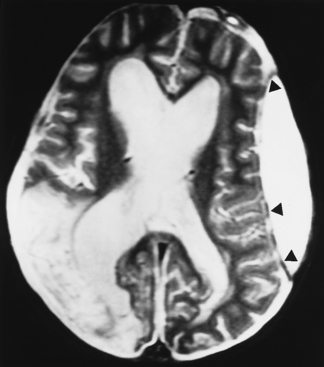
It is easy to be fooled on CT in patients with isodense subdural hematomas, particularly if the lesions are bilateral. In such a situation both lateral ventricles are compressed and may appear symmetric. There are two keys to this difficult diagnosis. First, look carefully at the sulcal size and note whether it is appropriate for the patient’s age. Large subdural hematomas usually occur in older patients, who should have prominent sulci. Anytime those sulci are poorly visualized, think subdural hematoma. Second, look at the gray-white interface. If this is buckled inward from its normal position, then consider an extra-axial mass (Fig. 5-15). Infiltrating intra-axial masses obliterate the interface. Contrast is helpful in isodense subdural hematoma by visualizing the inwardly displaced cortical veins. On call by yourself reading the ED CT and worried about an isodense subdural? Just ask for an enhanced scan.
On MR, subdural hematomas follow the intensity of blood just as epidural hematomas do (see above). (Fig. 5-16; see also Figs. 5-11 and 5-12). Chronic subdural hematomas rarely have significant amounts of hemosiderin deposition except after recurrent hemorrhages (Fig. 5-17).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree