Fig. 11.1
Classification of Congenital Scoliosis
Most common cause of congenital scoliosis.
Further subclassified as segmented, semisegmented, and nonsegmented (Fig. 11.1).
Segmented: still have growth plates both cranially and caudally
Semisegmented: fusion with a cranial or caudal vertebra. And there is a functional disc on one side only
11.2 Anatomy
A hemivertebra has a partial vertebral body, a pedicle, and a lamina
May be joined to the level above or below at the body, the hemilamina, or both. If the HV is not fused to either adjacent segment, there is higher potential for asymmetric spinal growth.
A local lordotic or kyphotic deformity may occur with a hemivertebrae, if the associated failure of formation is greater anterior or posterior, respectively.
11.3 Pathogenesis
Progressive spinal curvature due to a hemivertebra is a result of uneven growth.
HV morphology is frequently characterized by a wedge on the convex side of the scoliotic curve. In the presence of healthy growth plates above and below (fully segmented HV), the uneven growth on the convexity of the spinal curvature will generate progressive scoliosis.
The location of the partial vertebral body will generate different types of deformities. If the vertebral body lies in the posterolateral quadrant, a progressive kyphosis may arise and may or may not be associated with scoliosis.
The abnormal morphology secondary to the congenital defect can generate progression of a scoliotic deformity. Progression of the curvature is difficult to predict hence close follow-up through the child’s growth should be maintained.
In cases of progression, normally segmented areas of the spine become involved in the curve, causing increased deformity and spinal imbalance.
11.4 Natural History
Rate of deterioration and the ultimate severity of the curve depend on the type of anomaly, the age of the patient, and the location of the curve.
Defects at spinal transition levels such as the cervicothoracic and lumbosacral junctions produce more visible deformities.
Congenital spinal anomaly that has the potential to produce the most severe scoliosis is a unilateral bar with a contralateral hemivertebra, these are followed by unilateral bar, single hemivertebra, wedge vertebra, and finally a block vertebra which is the most benign of all congenital spinal anomalies [3].
Curve progression occurs more rapidly during the first 5 years of life and during puberty which represent the two main spinal growth spurts [4].
Progressive curvatures of the spine caused by a hemivertebra result from unbalanced growth.
Fully segmented hemivertebra have a much higher rate of progression, because the presence of an intact disc space above and below signifies the presence of active and potentially asymmetric spinal growth.
In a similar fashion, asymmetrical tethering of the spine leads to curvature with growth as is seen with bars or rib fusions on the concavity of a curve.
11.5 Patient Evaluation
Focuses on three areas: physical examination, the search for other anomalies, and radiographic evaluation.
Physical exam starts with height and weight to detect growth patterns.
Evaluation of the patient’s skin requires special attention to detect hairy patches, abnormal pigmentation, or skin tags over the spine. Early detection of spinal dysraphism can avoid missing treatment options prior to neurological impairment.
Lower extremity anomalies, such asymmetric calves, cavus feet, clubfeet, and vertical talus, should be ruled out.
11.6 Imaging and Work Up
Standing 36-in. posteroanterior (PA) and lateral radiographs are critical to define the deformity and assess Cobb angles.
Routine MRI of brainstem and full spinal cord before any surgical intervention given that 30–40 % of congenital scoliosis present with spinal dysraphism.
CT scan with three-dimensional (3D) reconstructions are required to delineate the anatomy of anterior and posterior elements, prior research has demonstrated that CT reconstructions are accurate in predicting intraoperative findings [5]. It helps anticipate possible intraoperative problems such as posterior element deficiencies and fusions.
Pediatric radiology protocols should be implemented to reduce radiation exposure.
Renal and genitourinary system ultrasound and cardiac echocardiography should be included to rule out VACTERL associations.
11.7 Differential Diagnosis
Failure of vertebral formation.
Failure of vertebral segmentation.
Sequela of infection causing partial vertebral body destruction.
Tumor
Vertebra plana/eosinophilic granuloma
11.8 Nonoperative Treatment
Reserved for nonprogressive curves caused by HV. Unsegmented or partially segmented HV may be closely followed during growth with radiographs every 6–12 months, depending on the degree of the deformity and age of the patient.
11.9 Surgical Treatment
11.9.1 Preoperative Planning
Review MRI imaging
If spinal dysraphism is present, referral to neurosurgery is warranted. If decompression, detethering, or any other neurosurgical procedure is required, it should precede HV resection either same setting or in a staged manner.
Review 3D CT scans (Fig. 11.2).
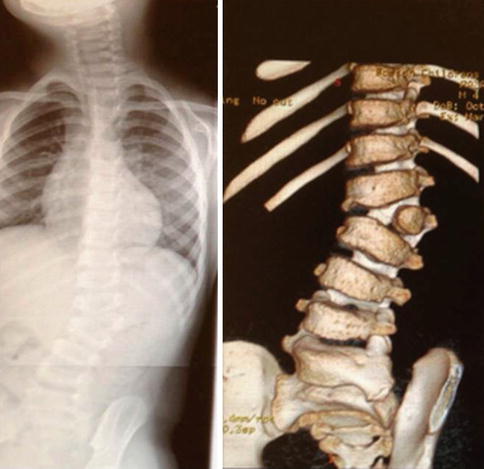
Fig. 11.2
X-ray and 3D CT scan reconstruction: 4-year-old male L2 fully segmented hemivertebrae
Complete understanding of anatomy of the HV since posterior element fusions or absences can make identifying levels difficult.
Pedicle anatomy, dimensions, and orientation of the levels above and below is mandatory to plan fixation methods.
Neurologic monitoring with somatosensory and motor-evoked potentials.
Ensure frequent communication between neurological monitoring and anesthesia teams to help prevent neurological injuries
11.9.2 Positioning
Prone position in radiolucent operating frame with chest and pelvic support to leave abdomen free.
Apply bolsters or slightly “airplane” the table so the convex side is slightly higher than the concave side. This help with anterior visualization, bleeding, and retraction of the dura and its contents.
Before draping, place a marker over HV region and obtain a radiograph. This helps confirm the level of the hemivertebra and avoids mistakes in the approach, which can be confusing because of the abnormal anatomy.
If simultaneous anterior–posterior procedure is planned, place the patient in lateral decubitus.
This approach is recommended when a medical condition caution against excessive bleeding, when extreme lordotic components render posterior access to the vertebral body difficult, and when the surgeon is unfamiliar with posterior-only approaches to circumferential surgery.
Position convex side up and slide the back to the edge of the table to facilitate posterior retractor placement.
11.9.3 Approach
Anterior–posterior approach should be standard transthoracic, transthoracic–retroperitoneal, or retroperitoneal depending on location of the HV. Approach is usually limited as it only involves exposure of the HV and the disc above and below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








