Fig. 1
Physiological parameters after surgical procedures up to 120 min after SAH (a). The data before SAH are not shown. There are significant differences between the SAH hypotension and SAH BP preservation groups in heart rate and mean arterial blood pressure after SAH (* p < 0.001). Values are mean ± SD. Severity of SAH scores demonstrated equivalent SAH severity between SAH hypotension and SAH BP preservation groups (b). Values are expressed as median and 25th to 75th percentiles
Quantification of Cleaved Caspase-3/Caspase-3 Ratio at the Apex of Heart
Western blot analysis was used to quantify the levels of cleaved caspase-3/caspase-3 (CC3/C3) ratio in the sham (n = 4) and SAH rats (n = 8), including both the SAH hypotension and SAH BP preservation groups. The levels of CC3/C3 ratio in SAH group were 4.9 times higher at the apex of the heart compared with the sham group (Fig. 2a, b, p < 0.05). Next, the levels of CC3/C3 ratio were quantified in the sham (n = 4), SAH hypotension group (n = 4), and SAH BP preservation group (n = 4). The SAH hypotension group had significantly higher CC3/C3 ratio compared with the sham as well as the SAH BP preservation groups (Fig. 2a, c, p < 0.05).
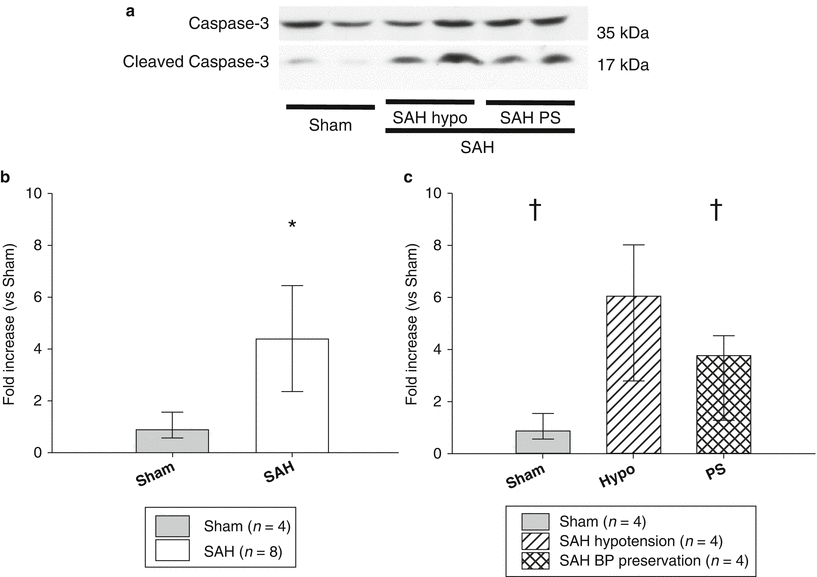

Fig. 2
Representative Western blots analysis 2 h after the surgical procedure in sham, SAH hypotension (hypo), and SAH BP preservation groups (PS) (a). In quantitative Western blot analysis, the SAH groups, including SAH hypotension (hypo) and SAH BP preservation groups, induced significantly higher cleaved caspase-3/ caspase-3 ratio than sham (b). The SAH hypotension group had significantly higher cleaved caspase-3/caspase-3 ratio compared with the SAH BP preservation group (c). Values are expressed as median and 25th to 75th percentiles. * p < 0.05 vs. sham, † p < 0.05 vs. SAH hypotension group
Immunofluorescence Localization of Cardiomyocyte Apoptosis at the Apex of the Heart after SAH
Immunofluorescence staining was performed in the sham, SAH hypotension, and SAH BP preservation groups to localize apoptotic cell death at the apex of the heart using antibody specific to the cardiomyocyte. α-Actinin is one of the essential structural proteins required for establishing cardiomyocyte elongation and coordinated muscle contraction [14]. TUNEL-positive cells were detected in the heart tissue, which co-localized with the α-Actinin-positive cells in the SAH groups (Fig. 3). In the left ventricular (LV) wall, SAH with hypotension increased the number of TUNEL-positive cardiomyocytes, which were relatively fewer in the SAH BP preservation group.
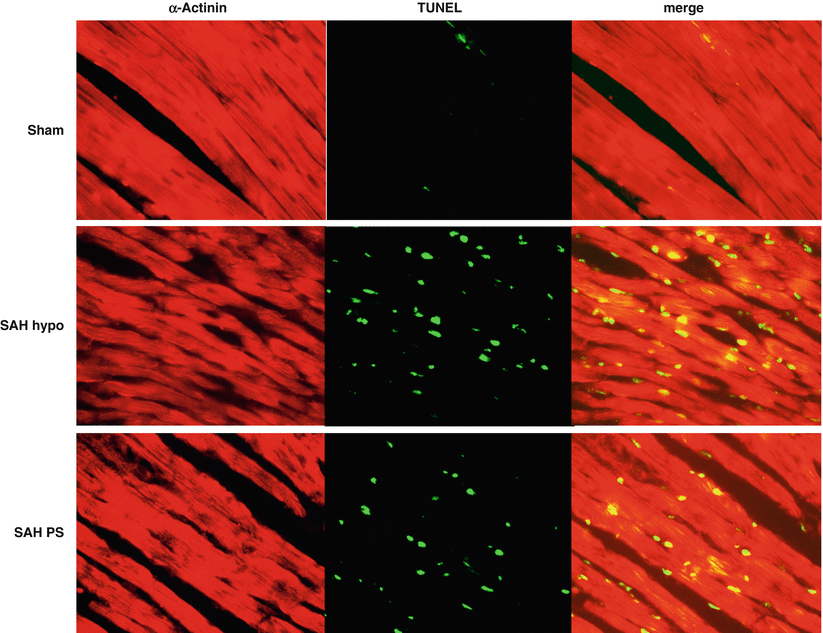

Fig. 3
Representative immunofluorescence of α-Actinin and transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling (TUNEL) staining in sham, SAH hypotension, and SAH BP preservation groups in the left ventricle. SAH with hypotension had more TUNEL-positive cardiomyocytes than SAH BP preservation
Discussion
Our results demonstrate that apoptotic cell death was detected in the heart at 2 h after SAH, and there was more apoptotic cell death in the SAH hypotension group than in the SAH BP preservation group, located on the cardiomyocyte in the LV wall.
Neurogenic stunned myocardium is the most severe form of cardiac injury after SAH [17]. Explosive increases in intracranial pressure can cause catecholamine release at the nerve terminal of the cardiac myocytes. As the heart muscles contract, adenosine triphosphate is depleted, which causes mitochondrial dysfunction and resultant myocardial cell death [11]. Although high levels of catecholamine have been shown to cause apoptotic and necrotic myocytes [7], it remains unknown whether heart failure after SAH is associated with apoptotic cell death in the heart. An intriguing syndrome that overlaps substantially with SAH-related cardiac dysfunction is tako-tsubo cardiomyopathy, also known as apical ballooning syndrome, stress cardiomyopathy, or “broken heart” syndrome [9]. Because we wanted to elucidate whether cardiomyocyte apoptosis is involved in acute heart failure after SAH, we measured BP and regarded the hypotension as a result of LV dyskinesis after SAH in this study. We detected cardiomyocyte apoptosis as a ratio of caspase-3 activation by taking the ratio of cleaved caspase-3 to caspase-3 because cleaved caspase-3 is the active form of the pro-apoptotic caspase-3 [3]. Our results suggest for the first time that cardiomyocyte apoptosis in the LV wall may cause the acute heart failure sometimes seen after SAH. Further studies are necessary for clinical translation in humans [1, 8, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








