Fig. 3.1
Meanders: a model for arterial remodelling. This photo of a Canadian Arctic river, taken from a float plane on the way to a canoe trip, shows that sand banks (plaques) build up in low-shear zones (where in arteries endothelin is expressed), whereas in the high shear zones (where in arteries nitric oxide is expressed), the river remodels away from the high shear (Courtesy of Dr. Ann Spence, an avid wilderness canoeist)
Strokes due to small vessel disease, on the other hand, are directly caused by high blood pressure. For this reason they occur in a particular distribution at the base of the brain—the ancient part of the brain that preceded evolution of large cerebral hemispheres, called by Hachinski [24] the “vascular centrencephalon.” In this region of the brain, short, straight arteries with few branches convey high pressure directly from the large artery to the resistance vessels, resulting in damage to the arteriolar wall. This leads to hyaline degeneration (Fisher’s “lipohyalinosis”) and fibrinoid necrosis, which in turn cause lacunar infarction when the arterioles occlude and hypertensive intracerebral hemorrhage when they rupture.
It is for that reason that true lacunar infarctions and intracerebral hemorrhages have a particular distribution—in the basal ganglia, thalamus, internal capsule, cerebellum, and brainstem [14, 25]. Figure 3.2 illustrates this concept. The cortex is supplied by long arteries with many branches, like a step-down transformer, so that blood pressure is much lower there. Lobar hemorrhages are due not to hypertension, but to amyloid angiopathy, or due to hemorrhage from lesions such as arteriovenous malformations or tumors.
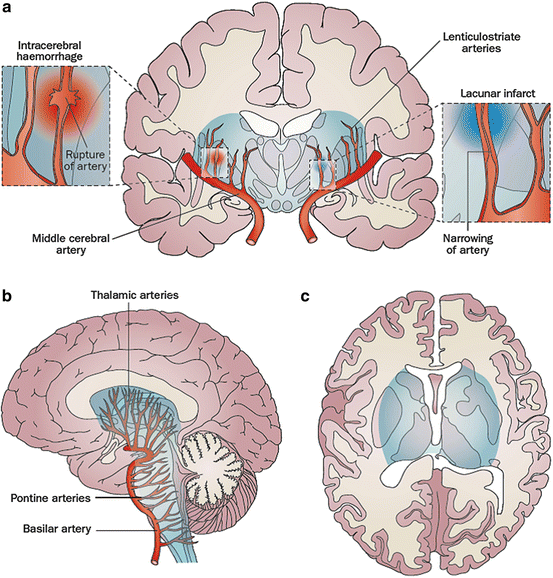

Fig. 3.2
Pathogenesis of lacunar infarction and hypertensive intracerebral hemorrhage. End arteries originate perpendicularly from the major vessels of the anterior and posterior circulation, with few branches, so pressure is transmitted directly from the large artery to the resistance vessels. Direct damage from high blood pressure causes hyaline degeneration, fibrinoid degeneration (and possibly microaneurysms) of the vessel wall, and results in narrowing (lacunar ischemic infarct) or rupture (intracerebral hemorrhage) of arteries. End arteries supply the vascular centrencephalon (blue region), which includes phylogenetically older parts of the brain (including the brainstem, basal ganglia and thalamus), and adjacent white matter. (a) Coronal view. (b) Sagittal view. (c) Axial view. Reproduced by permission of Nature Publishing Group from: Soros P, Whitehead S, Spence JD, Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol. 2013;9:174–8 [14]
Besides small vessel disease due to hypertension, lacunar infarctions have also been attributed to “micro-atheroma.” It seems likely that this pathogenesis is more important for small vessel disease in diabetes, which predisposes to intracranial stenosis [26].
2.1 Lacunar Infarction
The recent tendency to call small infarctions seen on imaging, regardless of their location, lacunes, impairs the understanding of the pathogenesis of stroke subtypes. In my view, the term “lacunar infarction” should be reserved for small infarctions due to occlusion of arterioles or their small branches, in the vascular centrencephalon. Many such infarctions, but not all, may correspond to one of the classic “lacunar syndromes” attributed to Dr. C. Miller Fisher: pure motor, pure sensory, dysarthria/clumsy hand syndrome, ataxic hemiparesis and mixed sensorimotor stroke.
The spectrum of hypertensive small vessel disease seems to be changing with better control of hypertension. Whereas in the past authors described lipohyalinosis as a cause of lacunar infarction, fibrinoid necrosis as a cause of lacunar infarctions and fibrinoid necrosis and microaneurysms [27, 28] as causes of hypertensive intracerebral hemorrhage, these findings are now rarely seen. Challa et al. [29] suggested that Charcot–Bouchard aneurysms may have been artifacts due to misinterpretation of slices of dilated arterioles, though Sutherland and Auer thought they had seen one in a report in 2006 [30]. We were not able to find a single case of a Charcot–Bouchard microaneurysm in the archives of our pathology department, and fibrinoid necrosis is now mainly a consequence of radiation. Figure 3.3 shows lacunar infarction in gross and microscopic views and examples of lipohyalinosis and possible fibrinoid necrosis.
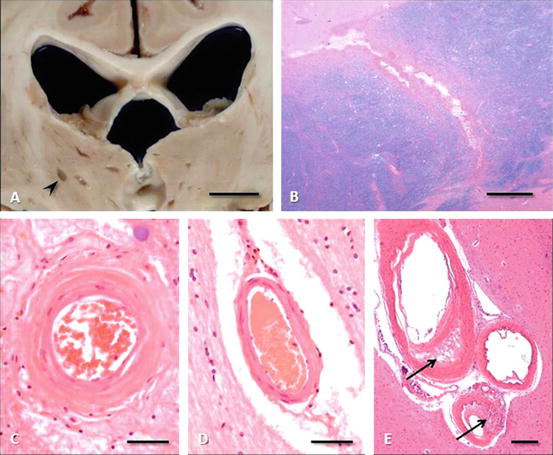

Fig. 3.3
Lacunar infarction. (a) Multiple bilateral thalamic lacunar infarcts (arrowhead) as seen in a coronal section at the level of the lateral geniculate nuclei (bar = 1 cm). (b) Lacunar infarct traversing the anterior limb of internal capsule between caudate (upper left) and putamen (lower right) (hematoxylin, eosin, and luxol fast blue stain, bar = 1 mm). (c) Basal ganglionic arteriole of a patient with chronic hypertension revealing the concentric mural thickening of arteriolosclerosis (hematoxylin and eosin, bar = 50 μm). (d) Normal lentiform arteriole for comparison (hematoxylin and eosin, bar = 50 μm). (e) Small cluster of basal ganglionic arterioles, two of which display changes of Fisher’s lipohyalinosis, with asymmetrical disruption of mural architecture and focal mononuclear/macrophage infiltrates (hematoxylin and eosin, bar = 250 μm)
2.2 Intracerebral Hemorrhage
Examples of hypertensive intracerebral hemorrhage are shown in Fig. 3.4, and contrasted with lobar hemorrhage, which is due to amyloid angiopathy. Whereas in the late 1970s we saw at Victoria Hospital ~200 hypertensive intracerebral hemorrhages per year, the commonest form of intracerebral hemorrhage in our hospital nowadays is lobar hemorrhage, which is unrelated to hypertension.
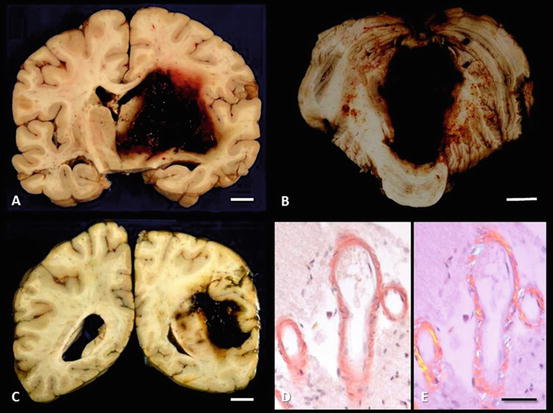

Fig. 3.4
Intracerebral hemorrhage. (a) Hypertensive hemorrhage, right basal ganglia, breaching the lateral and third ventricles and extending laterally to the insula. (b) Hypertensive hemorrhage, midline cerebellar white matter, compressing and breaching the fourth ventricle (c) Lobar hemorrhage from congophilic angiopathy. Mass effect of the hemorrhage and subsequent cerebral edema compressed the lateral ventricle and led to uncal herniation. (d) Congophilic angiopathy; congo red stain viewed under normal (left) and polarized (right) light
3 Stroke Prevention by Control of Hypertension
These distinctions explain the results of an “experiment of nature” that occurred in London, Canada, between 1978 and 1983. The Department of Family Medicine mounted a major program to detect and treat hypertension in the community [31]. By fortunate coincidence a hypertension clinic had opened there in 1977 [32], so patients whose blood pressure was not controlled by their family physicians could be referred for investigation and treatment. By another coincidence, the first CT scanner in the region was installed at Victoria Hospital in 1976, so it was then possible to distinguish hemorrhagic from ischemic strokes and to more accurately assess stroke subtypes. It is assumed that in 1978 blood pressure control in the community was as poor as elsewhere in North America: approximately half of cases detected, of those half (i.e., 25 %) on treatment, and of those, half, or only 12.5 % of the total, controlled. However, after 5 years, the level of blood pressure control in the community reached unprecedented levels: 94 % detected, 92 % on treatment, and 72 % controlled [33]. This resulted in a 50 % reduction of stroke; however, virtually all of the strokes prevented were those due to hypertensive small vessel disease: lacunar infarctions and intracerebral hemorrhages [34]. Unfortunately, such levels of blood pressure control have not been maintained, either in our community [35] or elsewhere. Nevertheless, even with imperfect blood pressure control, hypertensive intracerebral hemorrhages, which were almost a daily occurrence at Victoria Hospital in the mid-1970s, are now unusual—it is more common to see lobar hemorrhages from amyloid angiopathy.
All of the above indicates that what is most important about preventing strokes from hypertension is blood pressure control. Although much has been made about whether angiotensin-converting enzyme (ACE) inhibitors [36] or angiotensin receptor blockers (ARBs) [37, 38] might be more effective in reducing stroke, it depends on the population and perhaps on the proportion of patients in a given study who are of African origin. As discussed in 2006 [11], diuretics were the most effective drugs in the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) [39], with 40 % African-American participants, whereas in the Australian National Blood Pressure study (ANBP2) [40] and Anglo-Scandinavian Cardiac Outcomes Trial (ASCOTBPLA) [41], with fewer than 5 % black patients, ACE inhibitors and calcium antagonists were the best drugs.
To be sure, angiotensin receptor antagonists [42] and angiotensin-converting enzyme inhibitors [43, 44] have beneficial effects on the heart, kidneys, and vasculature independent of blood pressure. However, aldosterone antagonists have similar beneficial effects on the arteries and the heart in hyperaldosteronism [7, 45]. It is important, therefore, in choosing antihypertensive therapy, to define the underlying physiology of the patient with regard to plasma renin and aldosterone [5]. The problem with consensus guidelines is that they tend to assume that all patients with hypertension should receive the same treatment. This approach is an important cause of uncontrolled hypertension.
Thus the key to stroke prevention, insofar as medical interventions are concerned, is control of resistant hypertension. The main causes of resistant hypertension are noncompliance, consumption of substances that aggravate hypertension (salt, licorice, nonsteroidal anti-inflammatory drugs, decongestants, and excess alcohol), and undiagnosed causes of hypertension that require specific treatment (secondary hypertension) [12]. The only NSAID that does not raise blood pressure is sulindac [46]. With the exception of rare causes such as pheochromocytoma and aortic coarctation [32], most cases of resistant hypertension are due to disorders of the renin-angiotensin-aldosterone axis and will be controlled by individualized therapy based on intelligent use of plasma renin and aldosterone profiling [12, 47]. This approach is of particular importance in black patients [5].
4 Treatment of Hypertension in Acute Stroke
The brain is encased in a rigid skull and divided into compartments tough fibrous structures—the dura mater (falx and tentorium), as well as white matter tracts that are quite firm, such as the corpus callosum, that are firm relative to normal gray matter. An increase in pressure due to either swelling following ischemia, or due to space occupation by an intracerebral hemorrhage, therefore raises pressure within a given compartment, such as the posterior fossa or, for example, the middle cerebral artery territory. Rising tissue pressure opposes blood pressure at the cost of perfusion, adding insult to injury. Besides the space occupation from the hemorrhage per se, a surrounding zone of edema ensues, adding further mass effect. There is often edema surrounding a sudden intracerebral hemorrhage—as if the brain has been punched. In ischemic zones, cerebral blood flow, which is normally autoregulated, becomes pressure passive (Fig. 3.5). In this setting, a drop in blood pressure reduces blood flow more than it normally would, and a high blood pressure increases blood flow more than it normally would; capillaries are exposed to high pressures from which they would normally be protected, with worsening of cerebral edema. Thus there is a U-shaped relationship between blood pressure and outcome in acute stroke. Both very high blood pressure and low blood pressure that drops below the required perfusion pressure result in worse outcomes.
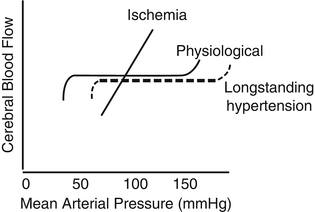

Fig. 3.5
Loss of cerebral blood flow (CBF) regulation during acute ischemic stroke. In physiological conditions, CBF is autoregulated over a wide range of perfusion pressures, from ~50- to 150-mmHg mean arterial pressure. This is shifted to the right in long-standing hypertension because of arteriolar hypertrophy. During acute ischemia, CBF becomes pressure passive, resulting in a marked reduction of CBF if pressure drops too low. The threshold at which this becomes a problem will be higher for patients with long-standing hypertension whose CBF autoregulation is shifted to the right. Reproduced by permission of Lippincott Williams and Wilkins from Spence JD. Treating hypertension in acute ischemic stroke. Hypertension. 2009;54:702–3 [59]
The problem of edema causing increased tissue pressure and gradually reducing perfusion of the viable penumbra is what accounts for malignant middle cerebral (MCA) infarction, and the marked benefit of hemicraniectomy for that condition: a doubling of survival and a doubling of survival without severe disability (a modified Rankin score ≤3) [48]. A key problem with this treatment is that the team managing the patient with malignant MCA syndrome must have the courage to move quickly to hemicraniectomy, before the edema has begun to strangulate the penumbra. Large ischemic areas on the diffusion-weighted MRI early in the course of ischemic stroke should lead to hemicraniectomy without delay, particularly in younger patients with little atrophy, and thus lacking space for expansion of the edematous tissue.
5 Intracerebral Hemorrhage
Although the notion that lowering blood pressure should limit growth of the intracerebral hemorrhage is attractive, there is little evidence that blood pressure lowering accomplishes that objective. In the recent Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT2) [49], the reduction of hemorrhage volume was only 1.4 mL and there was no significant reduction of death or severe disability. There was, however, an improvement of functional outcomes as assessed by the modified Rankin score. The editorial [50] accompanying the paper concluded as follows: “…given that INTERACT2 showed a trend toward a reduction in the primary outcome of death or severe disability, significant improvement in secondary functional outcomes, and reassuring safety data, acute blood-pressure reduction to a target systolic blood pressure of 140 mmHg or less appears to be a reasonable option for patients with spontaneous intracerebral hemorrhage.” Further studies of this problem are under way.
Hemicraniectomy has been less successful for intracerebral hemorrhage than for malignant MCA syndrome, but some small studies [51–53] suggest that it may be worth further study. Indications are that treatment must be early to be effective. The latest guideline from the American Heart Association is from 2007 and overdue for updating. It says there is insufficient evidence to support hemicraniectomy.
6 Cerebral Ischemia
There is undue controversy over the question of whether high blood pressure should be lowered in patients with acute ischemic stroke. Fear of aggravating the stroke by lowering blood pressure too rapidly and to levels that are too low is well founded, but largely due to poor treatments such as “sublingual” nifedipine or intramuscular hydralazine, that cannot be controlled once administered [54].
In some patients with acute stroke who have coexisting conditions such as aortic dissection, myocardial ischemia and pulmonary edema, the blood pressure must be lowered [55]. One such example seen by JDS is an aortic dissection that occluded both the carotid artery origin and a renal artery origin, with severe renovascular hypertension. The question in such cases is not whether blood pressure should be lowered, but how. Infusions of short-acting drugs that can be titrated carefully, or nitrate paste [56], which can be wiped off if blood pressure is dropping too low, are preferred to oral or intramuscular drugs, which once administered cannot be retrieved [57].
Because thrombolysis for acute ischemic stroke is contraindicated when blood pressure is above 185/110 [58], lowering of blood pressure to permit thrombolysis is increasingly common [59]. The 2007 AHA guideline states the following: “It is generally agreed that patients with markedly elevated blood pressure may have their blood pressure lowered. A reasonable goal would be to lower blood pressure by ~15 % during the first 24 h after onset of stroke. The level of blood pressure that would mandate such treatment is not known, but consensus exists that medications should be withheld unless the systolic blood pressure is >220 mmHg or the diastolic blood pressure is >120 mmHg” [58].
The difficulty with the latter part of this recommendation is that it assumes that all patients are the same; however, as shown in Fig. 3.5, patients with longstanding severe hypertension will have a rightward shift of their cerebrovascular autoregulation curve due to hypertensive arteriolar hypertrophy. They may tolerate such high pressures, whereas a person with no history of hypertension may not. I have seen hypertensive encephalopathy at blood pressures such as 180/100, in young patients with previously normal blood pressures who have a sudden rise in blood pressure to unaccustomed levels.
Solid evidence from randomized trials is not available, but there is some evidence informing this issue. Geeganage and Bath [60] analyzed 37 studies of blood pressure reduction in acute ischemic stroke. They reported that the lowest risk of death or dependency occurred in patients with blood pressure reductions of 14–15 mmHg. Large decreases or increases in blood pressure were associated with poor outcomes. Similarly, Sare et al. [61] reported that high systolic pressures, a small drop in systolic pressure, and large variability in systolic pressure were associated with poor outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








