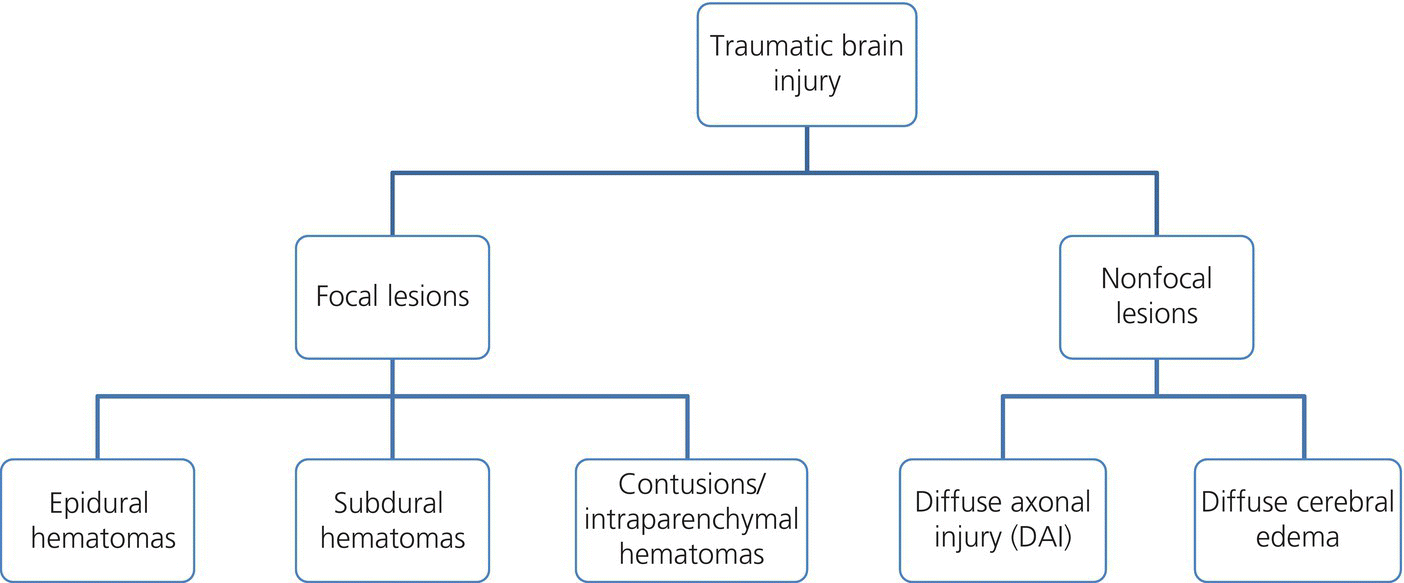
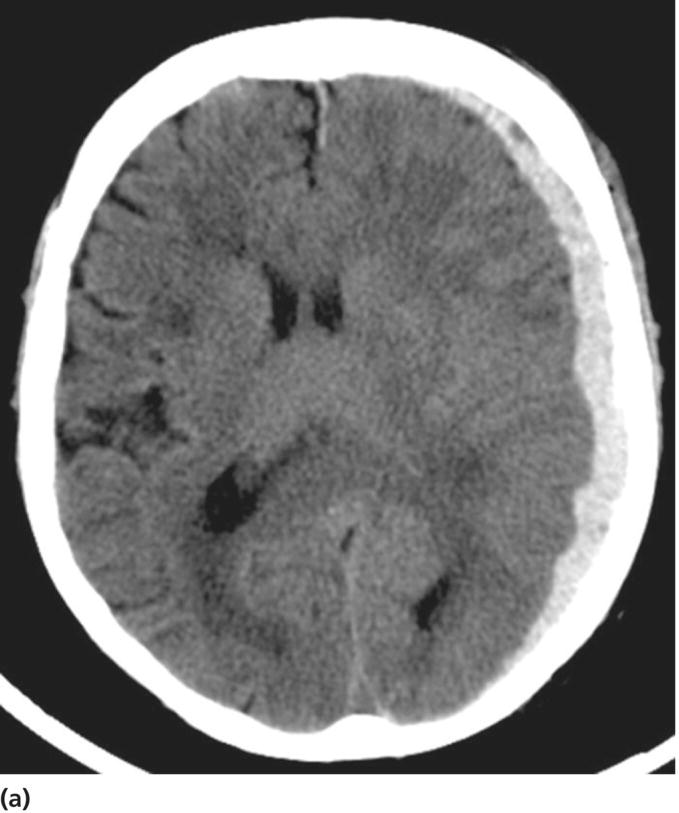
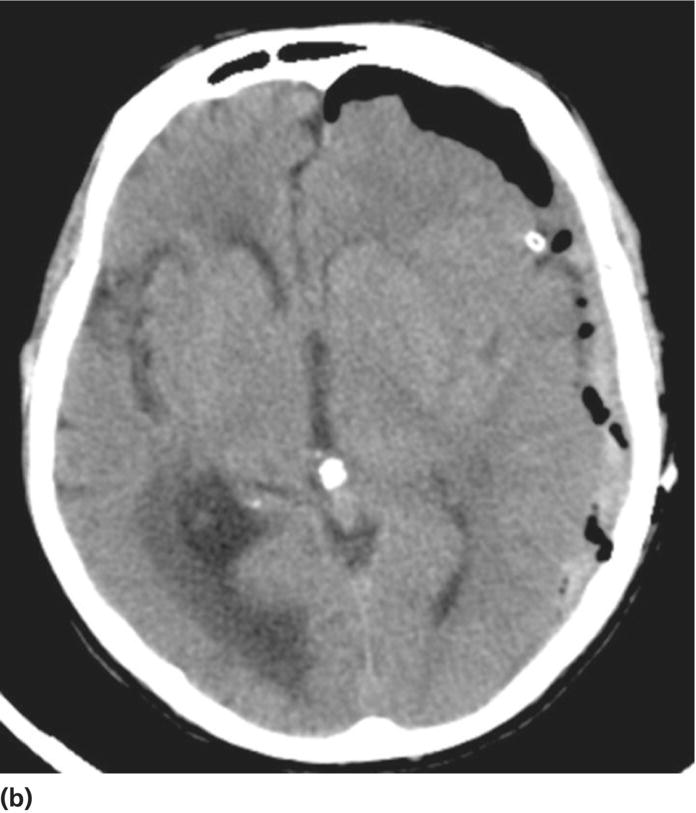
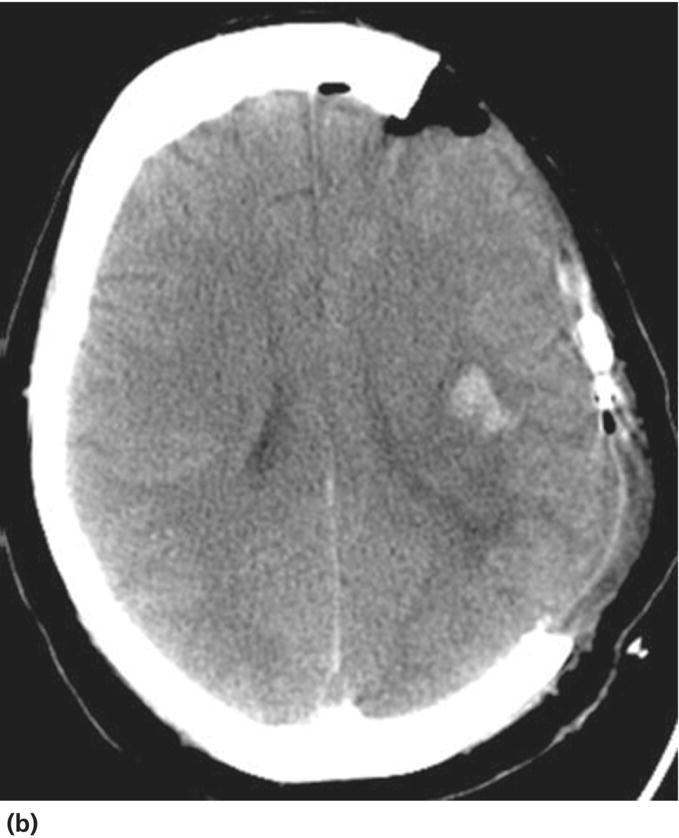
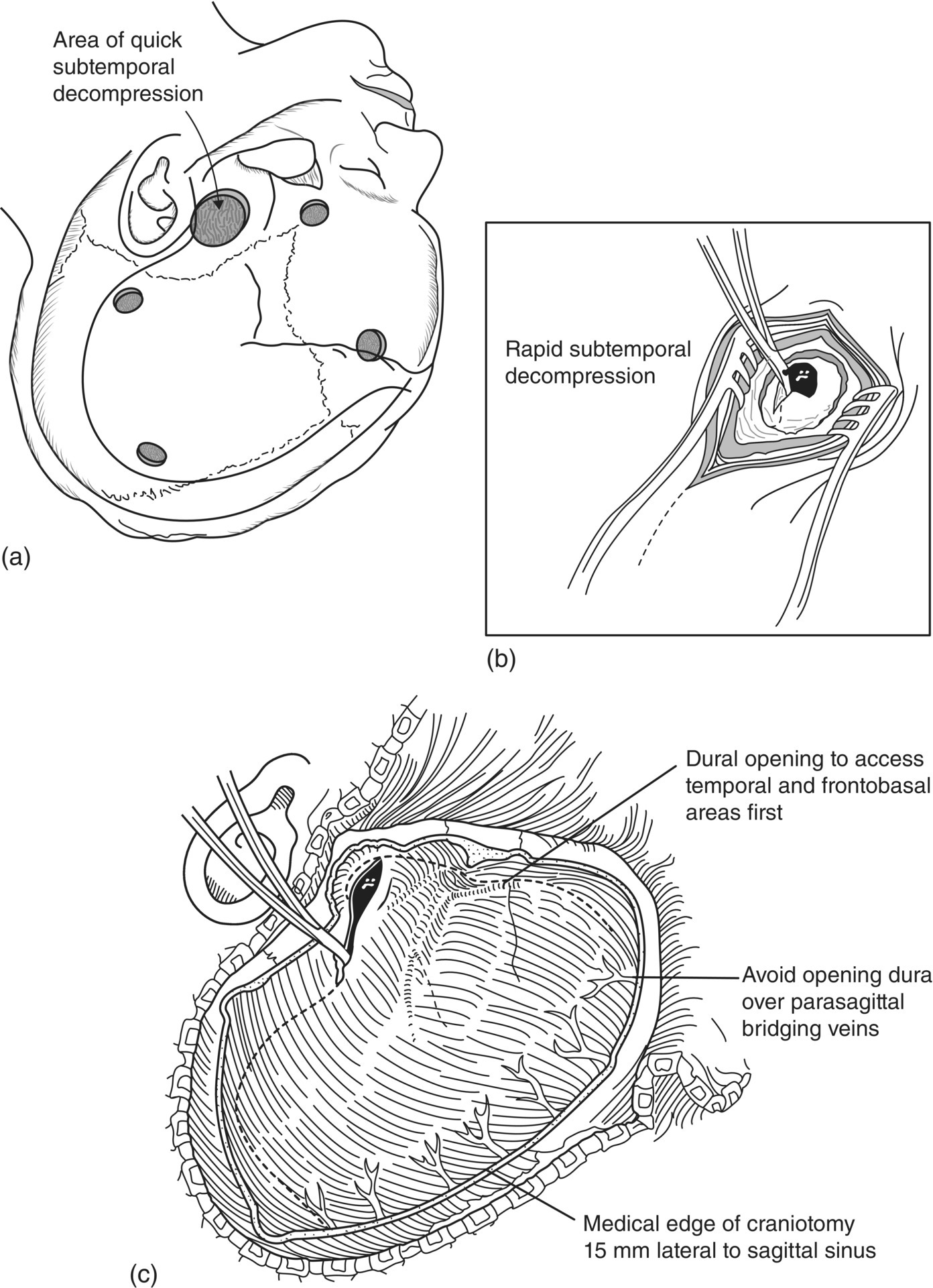
Chapter 6 Peter S. Amenta and Jack Jallo Department of Neurosurgery, Thomas Jefferson University Hospital, Philadelphia, PA, USA Within the industrialized world, traumatic brain injury (TBI) remains the most common cause of death in young adults, accounting for up to two-thirds of in-hospital deaths [1]. The overall mortality of trauma patients is three times higher in those afflicted with head injury in contrast to those without intracranial trauma. Death is attributed to the brain injury in 67.8% of cases [2]. Of those that survive, many suffer from poor quality of life and others fail to return to the same level of productivity as preinjury. Despite the magnitude of this devastating disease, treatment options remain limited. The evolution in the understanding of the physiology and pathology of TBI has generated a shift in the manner in which this patient population is viewed and treated. Previous segregation of patients into operative and nonoperative categories has been replaced by a multidisciplinary approach in which medical and surgical interventions represent tools in a broader arsenal of general critical care. Although still a key component, neurosurgical intervention must be balanced with nonsurgical treatment alternatives. Medical therapy commonly comprises a major component of patient management. As a result, an understanding of surgical options is important not only to the neurosurgeon, but also to the neurologists and critical care specialists involved in the care of TBI patients. This paradigm shift has led to increased reliance of medical decision making on evidence-based practices. The decision to operate, the indications for surgery, the approach used, the efficacy of alternative therapy, and the ultimate goal of surgery are all open to discussion. Within the current medical environment, each of these variables is examined to uncover the scientific data that support or contradict chosen interventions. Unfortunately, the nature of TBI does not lend itself easily to randomized prospective analysis and current approaches are largely based on decades of clinical experience. There are multiple clinical scenarios in which intervention is obviously warranted, but little evidence-based data exist to define whether that intervention is medical or surgical. Neurosurgeons routinely employ operative intervention for clinical signs of herniation or impending devastating neurologic compromise, but no class I evidence exists to support these decisions. Further complicating the management of TBI are those patients with a normal and stable neurologic exam, but imaging that reveals what was traditionally viewed as a surgical lesion. As evidence-based practice continues to evolve, the literature must begin to answer these questions to optimize the management of this patient population. The modern understanding of TBI and the role of surgical management begins with the publication of the findings of the International Coma Data Bank in 1975 [2]. This multicentered prospective analysis of 1000 comatose patients provided the data that made possible the Glasgow coma (GCS) and Glasgow outcome (GOS) scales. Building upon these data, the National Traumatic Coma Data Bank examined multiple clinical variables, including the quality of survival following severe head injury, the utility of measuring intracranial pressure (ICP), the impact of aggressive preadmission and emergency room care, and the identification of early predictors of clinical course and ultimate outcome [3]. The 1991 Traumatic Coma Data Bank represents one of the first attempts in the literature to define the role of surgical intervention in the management of TBI. The findings of this large multicenter data set highlighted the benefit of the evacuation of intracranial mass lesions, the importance of controlling ICP, and the deleterious effects of preadmission hypoxia and hypotension [4–6]. Based on these data, the Guidelines for the Management of Severe Traumatic Brain Injury was initially published in 1996, yet this comprehensive review of the literature provided no guidelines for surgical management [7]. It was not until 2006, with the release of the Guidelines for the Surgical Management of Traumatic Brain Injury that the role of surgery within the multidisciplinary approach to TBI was addressed [8]. This extensive review of the literature did not identify any data higher than class III evidence pertaining to the surgical management of five commonly encountered traumatic injuries: acute epidural hematomas, acute subdural hematomas, traumatic intraparenchymal lesions, posterior fossa mass lesions, and depressed skull fractures [8, 9]. The data do not represent well-proven standards of care, but instead are a collection of surgical options that provide a basis upon which to build clinical decision making. As a result, the importance of training, comfort level, and previous experiences and complications cannot be underestimated. The following discussion is designed to feature the practice options detailed in the Guidelines for the Surgical Management of Traumatic Brain Injury, while also detailing important aspects of the pathophysiology of specific injuries. The information presented is intended to be of use to all practitioners involved in the care of the TBI patient. Additionally, neurosurgical intervention is highlighted within the broader context of a multidisciplinary approach to this patient population. TBI is best understood as a complex process that is composed of overlapping phases, including primary injury and its evolution, secondary injury, and recovery [10]. Primary injury represents a large category of insults that may be caused by multiple mechanisms, including, direct contact of the brain with the skull and shearing of the parenchyma as it stretches relative to the skull and dura [11]. This injury occurs at the time of insult and is directly proportional to the magnitude of the force applied to the brain. As a result, primary injury cannot be modified and is not amenable to treatment, but rather prevention. However, an accurate initial assessment of the primary injury is of the utmost importance as it assists in the determination of prognosis and overall outcome. Secondary injury is defined as the constellation of cellular and biochemical processes that are set in motion by the primary injury and then evolve over the subsequent hours and days [12]. Many of these processes have been identified to include breakdown of the blood–brain barrier, release of excitatory amino acids, oxidative stress, and shifts in ion gradients [13, 14]. These changes manifest clinically as cerebral edema, increased ICP, and eventual cerebral infarction and neurologic deterioration. For these reasons, secondary injury is a significant contributor to posttraumatic neurological disability, and its prevention has become the focal point of the medical and surgical care of TBI patients [10] (Figure 6.1). Figure 6.1 Phases of TBI (Data from Cernak [10], Lighthall and Anderson [11], McIntosh et al. [12], Lobato et al. [13], and Woie et al. [14]). For those patients with an operative lesion at first presentation, rapid triage is coupled with emergent preoperative preparation. Labs are drawn and processed, including blood type and cross-matching, platelet count, and PTT/INR. The medication profile, with special attention to antiplatelet agents and anticoagulants, is reviewed and prompt reversal of these medications must be immediately initiated. The preoperative evaluation begins with an assessment of the standard ABCs of trauma care. If the GCS is 8 or less, the airway is secured via endotracheal intubation. If there is evidence of acutely increased ICP and incipient herniation, temporary moderate hyperventilation provides transient reduction until more definitive measures may be employed. However, prolonged hyperventilation may result in reduced cerebral blood flow and resulting ischemia. Medical intervention should be maximized in the moments before the definitive procedure in the operative theater. For patients without contraindications to being seated in the upright position, the head is positioned well above the heart with the neck in neutral position to maximize venous outflow from the head. In addition to temporary hyperventilation, a majority of tertiary care institutions routinely initiate hyperosmolar therapy with intravenous mannitol (0.25–1 g/kg), normal saline, and furosemide. The indications for placement of an ICP monitor have been detailed elsewhere and therefore will not garner further discussion. The authors routinely employ the use of ventriculostomy in situations where it may be placed accurately. In cases of medically refractory ICP, sedation, and paralysis, including the use of barbiturates may be used as a temporizing measure until surgical intervention. It is important to include a determination of quality of life and salvageability in the preoperative evaluation of a patient with traumatic head injury. Family counseling and an informed discussion of prognosis are critical in this period, and factors such as advanced age, multiple medical comorbidities, and severity of intracranial and other traumatic injuries must be weighed when making the decision to proceed with surgical intervention (Box 6.1). Extra-axial traumatic intracranial lesions, such as epidural and subdural hematomas, are among the most common pathologies seen in tertiary care centers. Management of these lesions is dependent upon multiple factors including some irreducible degree of clinical intuition. In patients with neurologic compromise and an intracranial mass lesion, the general assumption and accumulated clinical experience has been to surgically evacuate the lesion as quickly as possible. However, the guidelines are much less clear in neurologically intact patients or in those with only minimal neurologic findings [5]. Recent investigations into the management of extra-axial lesions have provided a set of guidelines for the nonoperative treatment of these hematomas. Fully conscious patients with extra-axial hematomas may be treated conservatively with close observation when imaging shows the hematoma to be less than 10 mm at its thickest point and to be the single dominant lesion. Imaging studies must also show no features of mass effect, such as midline shift greater than 3 mm or effacement of the basal cisterns (Box 6.2) [15, 16]. Epidural hematomas are found in 6% of patients presenting with traumatic head injury and are often associated with an overlying skull fracture [17]. Regardless of the source of hemorrhage, epidural hematomas develop when blood accumulates between the periosteal layer of the dura matter and the inner table of the skull. The classic description is that of a skull fracture severing the anterior or posterior branch of the middle meningeal artery, resulting in a high-pressure arterial bleed in the temporoparietal region. Arterial sources of epidural bleeding are unlikely to tamponade and the natural history of these hematomas is to continuously expand. Epidural hematomas may also arise from a ruptured vein or venous sinus. Although venous bleeding is under relatively low pressure and likely to tamponade without intervention, arterial and venous hemorrhage cannot be reliably differentiated by current imaging techniques. Thus, all epidural hematomas must be assumed arterial in nature and treated accordingly. The widespread availability and speed with which a CT scan can be obtained provides an efficient and reliable imaging modality by which to diagnose an epidural hematoma. These intracranial lesions will most commonly appear as biconvex hyperdense lesions bounded by the dural insertions into the cranial sutures. Up to 5–10% of these lesions can lie both above and below the tentorium. Surgical decompression of an epidural hematoma has been found to be one of the most cost-effective of all procedures in terms of quality of life and years of productive life preserved [18]. This finding is due to the protection of the brain by the dura mater and the resultant relatively low incidence of underlying parenchymal injury. The prognosis associated with an epidural hematoma is largely dependent upon the neurological exam and level of consciousness at the time of surgery. Additional prognostic factors, such as age, time from injury to treatment, immediate coma versus lucid interval, presence of pupillary abnormalities, and postoperative ICP also influence neurologic recovery. A number of imaging characteristics found on CT at the time of presentation may also assist in determining prognosis and include hematoma volume, degree of midline shift, and the presence of an intradural lesion [19]. The resultant overall mortality rate for patients presenting with an epidural hematoma is 9% [17]. Craniotomy and evacuation of an epidural hematoma have long been the gold standard of treatment, yet the 2006 Guidelines for the Surgical Management of Traumatic Brain Injury were unable to identify any evidence greater than class III data to support surgical intervention. The study group was, however, able to provide a core set of treatment recommendations upon which to base current practices. The volume of the hematoma is one of the strongest indicators for surgical intervention and a supratentorial epidural hematoma greater than 30 mL should be surgically evacuated regardless of the GCS score. In the infratentorial compartment, any epidural hematoma with a volume greater than 10 mL should be evacuated. Hematoma thickness greater than 15 mm and midline shift greater than 5 mm as measured on CT were also found to be indications for surgical evacuation. Additionally, the study supported rapid surgical evacuation in patients with a GCS ≤ 9 and anisocoria. Multiple studies have also supported surgical evacuation of an epidural hematoma when found in the presence of additional intracranial lesions. Craniotomy continues to be the preferred method by which to achieve complete evacuation of an epidural hematoma, although no data were found to sufficiently support a particular surgical option [5, 8, 20, 21]. The guidelines for conservative nonoperative management of epidural hematomas are represented in the following table. As with all disease processes, a consideration of the resources available to each individual practitioner must be factored into the decision for surgical intervention. While it is never desirable to perform unnecessary surgery, observation does risk neurologic deterioration beyond repair. When considering the relative speed and ease with which an epidural hematoma can be safely evacuated, many practitioners choose to err on the side of surgery to prevent such catastrophic events. In the instance where observation is chosen, the practitioner must be confident in the reliability in which serial neurologic exams and imaging studies can be performed within the institution [5, 18]. In most instances, the focal nature of epidural hematomas allows rapid evacuation via a limited incision and craniotomy. Craniotomy and clot evacuation are performed in the usual fashion with suction, irrigation, and resection of the hematoma. In cases of rapid deterioration, the first burr hole is placed over the thickest area of clot to promote immediate decompression. If there is suspicion for an associated subdural hematoma, the dura is opened and the subdural space is explored. In most instances, the bone plate may be replaced [5]. Acute subdural hematomas represent the most common focal intracranial lesions in patients with severe closed head injury [4]. These lesions are also among the most deadly, with an average 50–60% mortality rate [17, 22, 23]. The underlying traumatic event is age dependent, with those aged 18–40 years most commonly being the victims of motor vehicle accidents, while those aged 65 years or older are most frequently the victim of falls [24]. Subdural hematomas develop when blood accumulates between the cortical surface of the brain and the dura mater. The bridging veins that traverse the subdural space en route to the dural venous sinuses represent the most common source of hemorrhage. The venous tear occurs when the mobile brain accelerates relative to the fixed dura and skull following the traumatic impact. Low-pressure venous bleeding will frequently tamponade without intervention. Subdural hematomas may also result from lacerated cortical blood vessels or eruption of a cortical contusion into the subdural space. Regardless of the source, acute subdural hematomas appear as hyperdense crescentic lesions that cross suture lines. Up to 10% of acute subdural hematomas appear isodense on CT if identified within 72 h of the traumatic event due to a low hemoglobin concentration [25]. Whether isolated or in conjunction with additional lesions, subdural hematomas lead to secondary injury by direct compression of adjacent structures, induction of edema formation, suppression of regional cerebral blood flow, and elevation of ICP. In turn, prolonged intracranial hypertension results in a reduction of cerebral blood flow and oxygenation [26]. A significant mechanical force is usually required to produce an acute subdural hematoma, resulting in up to 50% of patients presenting with an associated intraparenchymal lesion. As a result, the underlying parenchymal injury may prove to be the decisive factor in the determination of the ultimate outcome. Age is the most important variable in the determination of outcome, with higher mortality rates in patients aged greater than 65 years [24]. CT findings, particularly hematoma thickness and midline shift, are also reliable predictors of outcome in acute subdural hematomas. Acute subdural hematomas approaching 18 mm in thickness and causing 20 mm of midline shift are associated with a 50% survival rate. The survival rate progressively declines as shift exceeds hematoma thickness, dropping to 25% when shift exceeds hematoma thickness by 5 mm. The survival rate is 0% for hematomas resulting in over 25 mm of shift [27]. The presence of underlying edema, contusions, and effacement of the basal cisterns are additional variables associated with a poor prognosis [17, 28]. Multiple studies have proposed protocols for the nonoperative management of acute subdural hematomas [16, 29]. These studies are composed primarily of patients with high GCS scores, as those at the lower end of the spectrum are frequently taken to the operating room based on convention. Servadei et al. published favorable outcomes in two-thirds of comatose patients with subdural hematomas treated with nonoperative management [30]. All patients in this series had hematomas less than 10 mm in thickness and less than 5 mm of associated shift, experienced no neurologic deterioration, and were followed with an ICP monitor. Two of the fifteen patients eventually required craniotomy for intracranial hypertension and evolving intracerebral lesions. When surgical evacuation of an acute subdural hematoma is necessary, the primary goal is the rapid evacuation of the life-threatening intracranial mass. With the realization that subdural hematomas frequently occur in combination with other systemic and intracranial lesions, large craniotomies often provide the best exposure for managing any pathology encountered. Adequate exposure should allow for rapid evacuation of the hematoma, grant access to most sources of bleeding, and, in the event of severe cerebral edema, provide room for the brain to swell. To assist in postoperative control of ICP, the bone flap is left out if the brain appears edematous (Figures 6.2 and 6.3). Figure 6.2 TBI: Focal and nonfocal pathology (Adapted with permission from Chestnut [9]. © Thieme). Figure 6.3 Acute subdural hematoma. A 76-year-old male status post fall from standing during a syncopal episode presents with progressive right upper and lower extremity weakness for 24 h. Admission head CT reveals an 11 mm left subdural hematoma with 6 mm of associated left-to-right midline shift (a). Patient was taken for left-sided craniotomy and clot evacuation (b). A subdural drain was placed at the time of surgery. Most patients with severe head injury and 25% of those with moderate injury develop contusions or intracerebral hematomas. The majority of intraparenchymal hematomas are found in either the temporal or frontal lobes and are contrecoup injuries caused by the impact of the brain against the skull. Parietal and occipital hematomas are usually the result of a direct impact over the site of the lesion. In up to 50% of cases,{2269} serial imaging reveals enlargement of the lesion over the first few days due to increased swelling or continued bleeding. Most of the increase in contusion volume occurs in the first few hours. In the absence of surgical intervention, intraparenchymal hematomas resorb in 4–6 weeks by macrophage phagocytosis and gliosis. The choice between evacuation and observation of intraparenchymal hematomas remains among the most difficult management decisions in the field of TBI. The Guidelines for the Surgical Management of Traumatic Brain Injury offers treatment recommendations regarding intervention [8]. Intraparenchymal hematomas are particularly well suited for nonoperative management. The lack of a margin between salvageable brain and the hematoma and the inevitable need for dissection of uninjured brain to access the lesion represent significant risks of iatrogenic injury. As a result, decision making must be based on neurological exam, imaging, and clinical suspicion of future deterioration and development of intracranial hypertension. Serial CT is a valuable tool in monitoring intraparenchymal lesions and may aid in initial nonoperative management. CT scans revealing evidence of mass effect, including cisternal compression and midline shift, may be indicative of the need for surgical evacuation. Perhaps the most important guide in operative decision making is the attainment of an accurate admission GCS coupled with reliable serial evaluation and early recognition of clinical deterioration. ICP monitoring represents a useful tool in the management of this patient population, yet a significant number of patients will exhibit delayed neurologic deterioration without an associated increase in ICP [31]. When operative intervention is chosen, the goals of surgery are rapid evacuation of the mass lesion, acquisition of hemostasis, relief of mass effect, and reduction in ICP (Figures 6.4 and 6.5). Figure 6.4 Traumatic intraparenchymal hematoma. A 55-year-old intoxicated female presents status post fall from standing with GCS 7. Admission INR 4.7 secondary to warfarin therapy for atrial fibrillation. Admission head CT reveals a 7 × 4 × 5 cm intraparenchymal hematoma with intraventricular extension and 7 mm of left-to-right midline shift (a). The coagulopathy was reversed with factor IX and FFP. The patient was emergently taken for left-sided decompressive craniectomy and hematoma evacuation (b). A subgaleal drain was left at the time of the procedure. Figure 6.5 Schematic representation of a standard trauma craniotomy. Skin incision and suggested locations for burr hole placement (a). The inset shows rapid subtemporal decompression through a limited skin incision and evacuation of an intracranial hematoma (b). The dural incision is pictured (hash lines) along with underlying bridging veins that empty into the superior sagittal sinus (c). This standard craniotomy allows access to most subdural, epidural, and intraparenchymal hematomas (Reproduced with permission from Prabhu et al. [32]. © Elsevier).
ICU care: surgical and medical management—indications for immediate surgery
Introduction
The pathophysiology of traumatic brain injury

Preoperative evaluation and preparation
Extra-axial lesions
Epidural hematomas
Acute subdural hematomas
Contusions and intraparenchymal hematomas
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








