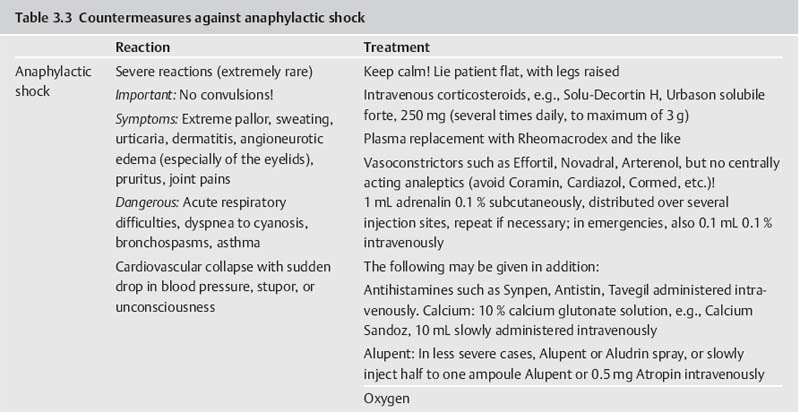
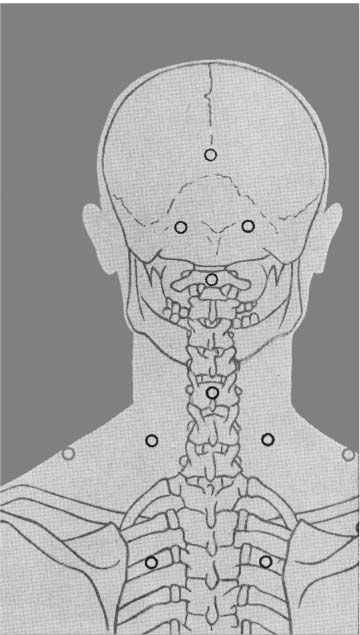
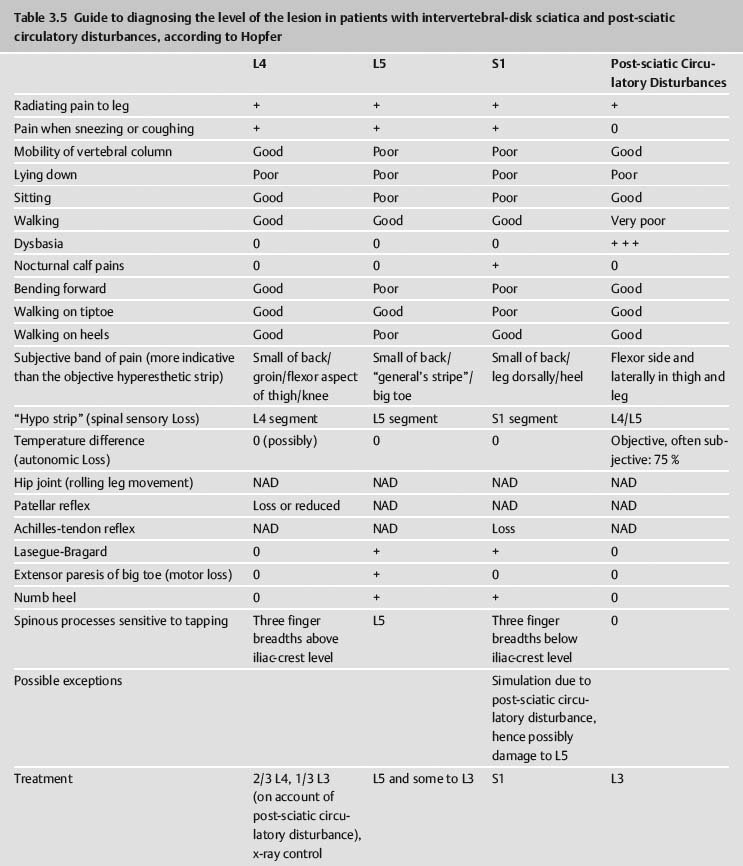
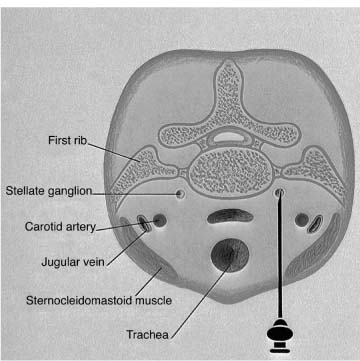
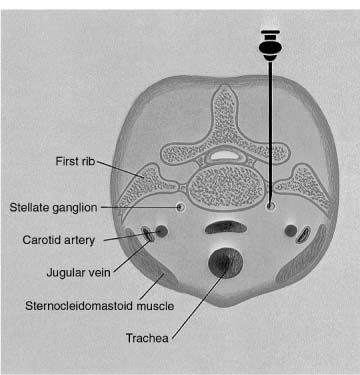
Part III 1. Neural therapy according to Huneke is a regulating therapy, i.e. a holistic therapy. The healing stimulus produced by means of a correctly placed neural-therapeutic substance produces a response from the whole of the neurovegetative system whose pathways are those taken by both illness and recovery. 2. Segmental therapy according to Huneke refers to the selective use of procaine or lidocaine in the area of the disease process. Always examine first, then test! The improvement achieved with segmental treatment increases with repetition up to complete cure. If segmental treatment fails to produce an improvement, look for the interference field. 3. Any chronic ailment can be due to an interference field. 4. Any part of the body can become an interference field. 5. The injection of procaine or lidocaine, repeated as necessary, into the responsible interference field will cure the disorder caused by it, as far as this is anatomically still possible, by means of a lightning reaction (Huneke phenomenon). 6. The conditions for a lightning reaction are: a. All disturbances remote-controlled from the interference field must disappear completely, as far as this is anatomically still possible, at the moment of the injection. b. Freedom from all symptoms must continue for at least 20 hours (8 hours in the case of teeth). c. If the disorder recurs, the injection (s) must be repeated, and the period of freedom from symptoms must clearly increase with every subsequent treatment. A Huneke phenomenon has been produced only if this criterion has been met. 7. If injection into the segment produces no substantial improvement, or an injection into a suspected interference field does not produce a 100% lightning reaction, further injections at these sites are pointless. 8. Always try simple injections with small quantities of local anesthetic first, with few but well-placed injections. Injections into the sympathetic chain and the ganglia are our last resort. A doctor who wants to help his or her patient must also be familiar with these. Do not stop treatment until you have tried everything. 9. All suspect teeth must be tested in a single session, similarly all scars. All scars in the same segment must always be injected as part of any segmental treatment. 10. NOTE: Intra-arterial injections into a vessel leading to the brain or into the subarachnoid space can have serious consequences. Always protect your patient and yourself by prior aspiration. → denotes that the key word following this sign is listed in the Alphabetical List of Conditions and Indications in Part II; → (T) denotes the key word following this sign is listed in alphabetical order in Part III, Techniques, where the technique for the injection may be found. Throw your heart across the hurdle and jump after it! Equestrian proverb The Huneke therapy offers many new therapeutic options that can improve the success of every practice. In order to take advantage of these options, one needs to master this therapy and learn how to apply the proper techniques in each individual situation without creating a general formula for the approach. This is not possible without some theoretical foundation. F. Huneke called his first book Krankheit und Heilung anders gesehen (Disease and Cure—a Different View); its new approach to pathogenesis and therapy needs to be adapted. Practicing neural therapy without taking a thorough case history and its analysis is half-hearted. We need the cooperation of the patient. Thus, a good relationship with the patient, thorough inspection, and particularly palpation, can complement the regular examination and help to find the clues needed for correct treatment. As students, we considered anatomy a boring and sometimes unnecessary exercise. Now it proves extremely useful when we have to familiarize ourselves with the location of nerves and vessels and the functions of the area that requires treatment. Every medical intervention contains the risk of failure, complications, and side-effects. They have to be recognized and eliminated before the beginning of treatment. The new techniques have to be studied. Before picking up a syringe, the location, direction, and depth of insertion as well as other aspects of treatment have to be clarified. Every beginning is difficult but this is worth the effort. Every practitioner who is convinced of the effectiveness of this healing art should follow the equestrian proverb stated above. He or she should follow their heart and overcome the obstacles. The obstacles are a combination of the psychological poison, which is fear, the hubris to trust one’s own abilities, and the weight of laziness and habits. With consideration, necessary caution, and courage, the physician should start out with simple injections. The best equestrian begins with small hurdles. I remember my first challenging injection well. I dared the attempt after a sleepless night and felt my heart pound in my chest while I was holding my breath. I remember my initial hesitation and my thoughts of giving up after the desired miracles did not occur immediately. Because there was no textbook available to me then, I decided to write one. Success comes with experience and one learns how to project the sensitivity for tissues onto the tip of the needle. I need to caution the daredevils, because they are in danger of violating the “Do no harm!” rule. We cannot harm the patient who comes to us with trust. We always need to apply our measures thoughtfully, adequately, and when necessary. Routine may never silence or replace the proper caution. The hesitant practitioners need to remind themselves why they became physicians—most likely to alleviate suffering. What if these injections are necessary to obtain this goal? The true physician will never avoid taking a chance for his or her own protection! “Taking a chance is the bow-wave to success” (Amery); recklessness is the same for failure. If this does not provide the required courage, they should at least educate themselves and refer to a capable neural therapist. I am always agitated by the ignorant statement that some of the injections that are listed here should only be performed by a specialist or an “experienced” neural therapist. How does one obtain experience? One must collect experience. This implies that one has to begin somewhere and sometime, which is the only way to gather experience. One has to work for it and cannot avoid difficulties or become discouraged by them. This is the only way to become a successful, experienced neural therapist. A good physician can do more good with a damp towel than a bad one with a whole chemist’s shop. Schweninger (Bismarck’s personal physician) Only close-fitting syringes must be used, and strong enough to stand up to considerable pressure. These should be absolutely sterile, carefully stored, and maintained in good condition, in accordance with standard practice. Disposable syringes are preferred. The following is a list of the different types of syringes that may be used by the neural therapist: 1. 2 mL record syringes: These are handy and easier to guide than bigger syringes, which also tend to tempt one all too easily to use unnecessarily large quantities. Disposable syringes have the disadvantage that due to their lightness they fail to give as clear a feeling of the needle position in the tissue as do the heavier syringes made of glass and metal. 2. 5 mL record syringes: Use only for injections where larger quantities are required. 3. Cartridge syringes: With longer and shorter needles for use in injections in the orthodontal area and for hard scars. Also very suitable for our purposes are all-glass syringes with the Luer-Lok attachment, and the three-ring syringe with the Recofix attachment available in 2 mL and 5 mL sizes. 4. The Dermo-Jet: Akra, a firm in Pau (France), has put a device called Dermo-Jet on the market. This injects the liquid under the skin almost painlessly, at high pressure and supersonic speed. It can be used for setting quaddles, through which one can then pass infiltrations at greater depth (e.g., into the sympathetic chain). The patient is unaware of what is being done and undesirable defensive movements are avoided. This instrument has proved useful particularly for children and nervous adult patients when large numbers of quaddles have to be set (alopecia, asthma, enuresis, pertussis). With this device it is possible to win over even the most injection-shy patient for neural therapy. A special attachment is also available for anesthetizing the injection sites for what is normally a somewhat painful dental test and thus makes this important investigation less unpleasant for both patient and doctor. There are four different models of the Dermo-Jet. For example, the Dermo-Jet Polymedical is a new improved model and has “Tip Jet” attachments, which are easily exchangeable and can be sterilized at 180°C and above, improving the protection against possible contamination through antigens. It is recommended that some of these attachments should be available in every practice. The use of the Dermo-Jet should be limited to children and overly fearful patients. When looked at microscopically, there is a considerable difference between quaddles that are set with an injection needle and those set with a Dermo-Jet. When set with the proper technique, the quaddles set with injection needles sit intracutaneously, which is directly subepithelial in the corium. Only a small portion of the Dermo-Jet quaddle is located in the corium. Due to the application of high pressure, the larger portion of the fluid reaches the subcutaneal area where it is dispersed. The Dermo-Jet can cause deeper tissue damage and microinjuries with increased bleeding. The microscope shows that the tip of an injection needle appears rather dull and arterioles can move out of its way, but they can be torn by the Dermo-Jet. Apart from size 1, 2, 12, 14, 16, and 18 needles, which are probably standard equipment in any surgery, we also require 80 mm, 100 mm and 120 mm-long needles, with a thickness of 0.8–l mm. Also recommended are 0.5 × 42 mm dental cannulas. For injection into the tonsils, the needle should have a short bevel. Disposable syringes are also available, with needles in all the lengths and thicknesses we require. In addition, there should also be a needle suitable for lumbar punctures, with a locking stilette. All the repeatedly used needles must be sharp and should therefore be replaced from time to time. Any needle whose point is bent over into a barb must be thrown away at once. When striking bone, the point of the needle may be bent over and form a barb, which can tear tissue when it is withdrawn or if it is used again. There have been reports of cases where nerve fibers have been damaged as a result of this. Since needles may break off where they have been soldered or attached to the adapter in some other way, they should never be pushed in “to the hilt.” Where deeper penetration is necessary, it is far better to choose a longer needle beforehand. For beginners, Strumann’s tonsil test needle is recommended, which is impossible to push in too far. For intravaginal injections, such as paracervical injections to Frankenhaeuser’s ganglia or to the pudendal nerve, the PP needle made by Woelm Pharma, Germany, can be recommended. Table 3.1 provides a list of needle size equivalents. 1. a neural-therapeutic product containing 1–2% procaine, or 0.5–1% Xylocaine or lidocaine (mepivacaine or prilocaine) solution for cases of procaine allergy; 2. Koch’s old tuberculin or Cutivaccine Paul-Novum for Ponndorf’s vaccinations; 3. vaccination fork, lancet, or needle, or ampule file, for Ponndorf’s vaccinations; 4. vaporizer for nasal spray and cotton swabs; 5. instrument sterilizer; 6. an examination couch that can be adapted for gynecological use, a chair with a headrest, good lighting; 7. tourniquet for intravenous injections; 8. reflex hammer, brush, needle, possibly a dress-maker’s tracing wheel, for testing reflexes and sensation; 9. grease pencil or felt pen for marking the skin; 10. surgical or disposable gloves; 11. bony skull and topographic atlas, for orientation prior to difficult injections; 12. oral spatula and pencil torch for treating the buccal cavity; 13. auriscope and nasal speculum; 14. test equipment for measuring skin resistance and finding acupuncture points (e.g., Svesa neural pen, by SVESA, Munich, Germany) and electrical skin test equipment (e.g., Mela Testator, by Mela Medical Ltd., Munich, Germany). Accidents can always happen, and the general practitioner must also be able to deal with these and have at least the minimum of equipment within easy reach and always ready for use. For this I suggest: 1. oxygen flask with pressure-reducing valve, Ambu artificial-respiration bag with face mask, Ambu Inc., USA; 2. pharyngeal and endotracheal tube, laryngoscope, tongue forceps, possibly also intubation equipment, electrocardiograph, defibrillator; 3. needles for intravenous therapy, preferably a self-retaining needle, ampoule file; 4. drugs in ampoules as follows: a. corticosteroids, such as methyl prednisolone, in doses of 50 mg, 250 mg, and 1000 mg; b. vasoconstrictors, such as arterenol, Effortil, Novadral, Sympatol (but on no account analeptics such as Cardiazol, Coramin, Cormed!), plus 0.1% adrenalin; c. plasma substitute, such as Rheomacrodex, with infusion equipment; d. for dealing with convulsions: valium, barbiturates such as Pentothal; only if artificial respiration is assured, relaxants such as Succinyl-Asta, Lysthenon; e. antihistamines, such as Antistin, Synpen, Tavegil; f. Alupent, 10% calcium glyconate. In essence, this is all we need by way of materials in order to practice neural therapy according to Huneke. Thus, there is no danger in our method that we may encounter everywhere else in medicine, of becoming dominated by our equipment. Quite the contrary! Neural therapy is a genuine part of the art of healing, in which creative medicine is fortunately still the most important element and the tools take second place. It is also a very young art, in which there is still a great deal of new territory to conquer. Naturally, we demand thorough general medical training as an essential basis and require every practitioner to make a searching preliminary examination of every patient, to exclude certain specific processes, together with properly responsible conduct at all times. We still learn more from life than from our teachers. E. von Bergmann Let me season what follows with an anecdote from my personal experience, in order to make what I have to say more palatable. As we know, even medicine is subject to fashions. At the start of the sulfonamide era, many a disciple of Aesculapius hastened to prove in well-founded scientific treatises that Prontosil worked genuine miracle cures in almost any internal and medical surgical disorders. Only a handful of doctors remained objective and adopted a wait-and-see attitude. Then it was Prontosil, yesterday it was penicillin, today it is corticosteroids, and tomorrow something else will be hailed as the wonder drug. I have no objection to progress. But the enthusiasm with which medicine greets each little step forward, with excessive praise and exaggerated expectations, and then has to backtrack, does tend to become just a little embarrassing after a while. As a young student I had the good fortune of being allowed to watch Kulenkampff at Zwickau during an operation. In the middle of it, the master pulled off his gloves, threw them on the floor and went with bare hands into the abdominal cavity, so that he might be better able to feel something or other in there. I was speechless with surprise and my aseptic conscience was appalled. “Anything the matter with you?” he growled at me, when I finally and audibly caught my breath again. My carefully phrased objection received the grumbling reply: “My young friend, remember that you can defecate (though he used a shorter and more profane term) into the abdominal cavity, you may spit into the thoracic cavity without fear of retribution, but you must not even peep into the knee joint!” As he was suturing the incision, I asked him whether he did not want to pour 50 mL of Prontosil solution into the abdominal cavity, as I had seen done elsewhere. His laughing reply was, at that time, something of a heresy, but today it seems wise: “No, why should I want to cover damaged and injured tissue with red dye as well?” This experience came to mind in 1951, when I was able for the first time to watch F. Huneke in his practice in Duesseldorf, trying to steal with my eyes as much as I could for my own practice. I experienced the same slight shock when I found that he never disinfected the patient’s skin before giving his numerous injections. His followers have adopted his approach. Millions of injections have shown that infections and injection abscesses hardly ever result. A survey produced only eight reports of infections in 35 000 000 injections. In some of these the cause lay in a therapy using corticosteroids or high-dosage regulation-blocking agents, which had substantially reduced the body’s defensive capabilities. In others we regard the resulting abscess as exacerbation of an old sealed-off infection and as a healing reaction. How does this come about? Is this to be interpreted to suggest that aseptic procedures are to be declared superfluous? There is no question of that, and for the benefit of critics who are not well-disposed to neural therapy let me emphasize that our syringes and needles must, of course, be sterile. Our experience with procaine has simply taught us to regard infection, and toxic and allergic reactions, from a new viewpoint. We explain the fact that even after injections under the scalp and into the oral mucosa no infection occurs, first of all by suggesting that the chemical and mechanical irritation produced by disinfecting the skin is perfectly capable of stirring the bacteria from a state of rest and making them virulent. And then, of course, we are injecting procaine. As long ago as 1906, Spiess noted that anesthesia suppresses any inflammation. We see danger not in the pathogenic agents but in the nerve irritation (depolarization) they cause. But we can reverse this with our anesthetic preparations, using them to stop bacterial and virus attack and proliferation, and thus preventing infection. If we infiltrate procaine around a fresh snake-bite, the venom can no longer act on the organism. This is explained first by the fact that the anesthetic breaks the conductivity of the nerve fibers. Thus, the nerves can no longer conduct the irritative stimuli to the nerve centers. But further, procaine is also capable of recharging the cell membranes damaged by the irritation and of restoring their normal electrical potential. By this means, the production of toxic stimuli is blocked, which would otherwise cause the center to respond with panicky, excessive, and therefore dangerous reactions. In tetanus, rabies, poliomyelitis, and many other diseases, we need to imagine similar processes taking place, reminding us of the interference-field theory and of the possibility available to us of eliminating the pathogenic nerve irritation with procaine. The picture is rounded out if in this connection we remember that serum sickness can be stopped by anesthetizing the serum injection site (Muschaweck). Far more important than all theory, however, is the fact that, whether we like it or not, that is the way it is. Obviously, no one can be prevented from continuing to carry out the traditional, ritual acts of ablution and of disinfecting the patient’s skin before injecting procaine. In certain circumstances, as, for example, before deep injections in the perineal region and near the anus, we also disinfect first. Similarly, for injections into the joints and the subarachnoid space and ventricles, the same sort of asepsis and antiseptic precautions must govern our actions as for major surgery! The same applies to seriously ill patients before parenteral treatment and to patients who are treated with high doses of corticosteroids. As has been stated, in return we need have no misgivings about being somewhat less punctilious in all other cases. T. C. Dann, in an article published in the Lancet, took the view that the standard few seconds’ routine skin disinfection before an injection is totally useless. At best, no more than about 80% of all bacteria are killed thereby. He and his colleagues had been giving injections for 6 years without prior disinfection, without ever finding any harmful side-effects result. They disinfected the skin only in above-mentioned exceptional cases. But in all such cases the skin is thoroughly cleansed for at least 2 (preferably 5) minutes with iodine, alcohol, or hexachlorophene. In 1978, Felig, in the Lancet, went so far as to describe the business of disinfecting the skin before injections as an “unnecessary ritual act.” In diabetics, where the risk of infection is substantially greater, 1700 injections were given without any prior disinfection of the skin around the injection site; not a single case of local or general infection resulted. My hobby? Impletol, of course! F. Huneke to a reporter Novocaine is the registered trade name owned by Hoechst Pharmaceuticals for the p-amino-benzoyl diethyl-aminoethanol hydrochloride discovered in 1905 by Einhorn. Its generic name is procaine. In earlier German and Soviet literature we generally find the name of Novocaine used, whilst British and American authors refer to procaine, the French to scurocaine. Procaine is thus an alcohol ester of p-aminobenzoic acid (PAB). It is hydrolytically broken down and thus detoxified via a serum enzyme, plasmacholinesterase, which occurs everywhere in the body tissues. This process takes from 20 to 40 minutes and produces two antihistamine components of interest: PAB and diethyl-aminoethanol. In the process of being broken down it is metabolized so thoroughly that only a small part (approximately 30%) of it needs to be detoxified in the liver and only 2% is eliminated unchanged via the kidneys. For this reason alone it is to be preferred for therapeutic purposes to a large number of more recent neural-therapeutic products based on an amide structure, such as Xylocaine, Scandicaine, Hostacaine etc., which need to be almost completely detoxified in the liver! Liver disease can lead to a reduction of the serum cholinesterase, because of which procaine will be metabolized more slowly. Other products such as butazolidine, chloramphenicol, and sulfonamides can delay detoxification. At this point, reference should also be made to the very rare congenital cholinesterase deficiency, which renders the patient incapable of metabolizing procaine. Apart from the neural-therapeutic action developed by the intact procaine molecule in pathologically changed tissue, there is also the effect of its breakdown products. PAB (vitamin HI) is regarded as one of the organism’s enzyme-building blocks. It acts as an intermediate stage in the formation of folic acid and of the citrovorum factor, which transmits the carbon-1 fragments in intermediate metabolism. PAB is probably also the main active agent against pathological sclerosing and hardening of the tissues. Diethyl-aminoethanol is a vasodilator substance that acts on the circulation and lowers blood pressure. Its spasmolytic effect on tonically constricted vessels and its influence on the neurovegetative state in sympathetic and parasympathetic irritation has been proved. In addition, a mildly stimulant effect on the central nervous system and psyche is also ascribed to it. It stimulates hair growth and sometimes restores youthful color to gray hair. Procaine blocks cholinesterase, inhibits the formation of acetylcholine and the sensitivity to stimuli of the peripheral choline receptors. It suppresses histamine formation. As a beta-receptor blocker it eliminates the physiological and pathological reactions caused by stress and sympathicomimetics. It lowers the level of catecholamines in the blood (epinephrine, norepinephrine, dopamine). For procaine and the other anesthetics, a number of specific pharmacological effects have been proved, all of which are desirable from our point of view. According to these studies: 1. It acts to restore neurovegetative equilibrium, i.e., it can act either as a stimulant, increasing tonicity, or as a relaxant to reduce tonicity, depending on the patient’s initial state. 2. It acts to relieve pain. Here, in addition to its central and peripheral analgesic effect, there is also an anti-pyretic, anti-allergic, and spasmolytic element. As the pain disappears, the reactive inflammation also vanishes. By eliminating pain receptors the pain threshold is raised. When the injections are placed correctly, this effect lasts longer than the anesthetic effect, which indicates a decrease of pathogenic feedback. This suggests that the repolarization of the cell membrane during the anesthesia-hyperpolarization, with subsequent membrane stabilization, has a positive effect on the regulation mechanisms. 3. Its effect on the nervous system is made up of its ability to act simultaneously on the peripheral, the autonomic, and on the central nervous systems. It alters the functional state of the nervous system by reducing its lability, thus making it less sensitive to harmful stimuli. It is thus in a position to eliminate the state of shock of different origins and degrees of severity. If used correctly locally, it blocks pathogenic reflexes and reactivates the previously blocked neurovegetative system with its spontaneous healing capability. 4. It develops a therapeutic effect on all three components of the blood supply, i.e., the heart, the vascular system, and the blood. It has a regulating effect on the blood supply, is anti-allergic and a vasodilator, and reduces the permeability of the vascular walls: a. Around the heart it inhibits the stimulus formation and conduction, and acts anti-arrhythmically. It has an oxygen-conserving effect on the heart muscle. b. Microcirculation improves with the opening of arteriovenous anastomoses. Edema can drain and inflammations improve. In animal testing, edema was prevented when paws were treated with procaine before compressing them. c. Animal testing proved the antihistamine effects of procaine, particularly by affecting the acute serum shock and suppression of the Shwartzman reaction. The stimulation of sensitive fibers causes histamine secretion, which stimulates more receptors. The antidromic reflex causes further histamine secretion. This lasts for a short while because the secretion is limited. Initially, sympathicus stimulation causes a spasm of the arterioles, which turns into pathological vasodilation with edema formation, vascular bleeding, intense pain with limited mobility, and muscle dysfunction. The sealing effect of procaine on the capillary walls begins quickly, reaches its peak after 1 hour, and lasts for up to 4 hours. In animal testing using procaine, the Bezold-Jarisch reflex of the induced collapse (loss of blood pressure, slowing down of pulse and respiration) can be prevented. 5. It also acts on the smooth musculature. So, for example, it sensitizes the uterus with regard to the hormone of the posterior lobe of the pituitary. 6. It has a substantial influence on the formation and secretion of hormones and enzymes. 7. It stimulates diuresis. 8. According to Uri, it also acts “directly on those parts of the brain which are associated with the transformation of stimuli into sensations.” 9. It regularly and quite noticeably improves the patient’s general condition. This means that a whole series of interrelated and interactive functions and regulating systems are reactivated, of which, however, we are able to find only a limited amount of objective evidence. The altering and balancing effects on the autonomic system and the regulation of sensitivity and trophism seem to produce positive results that include the psychological condition. Since the performance of organs and tissues is dependent on the blood supply and thus on the supply of oxygen and nourishment, and on the removal of the waste products resulting from metabolic processes, there is an increase in performance either directly locally at the treatment site or indirectly by the elimination of interference fields. This is enough to explain a large number of successful cures. It has also been proved that procaine has an oxygen-economizing effect in living tissue. The activating effect produced on non-specific defense mechanisms by procaine injections (subcutaneous and intramuscular, less clearly in the case of those given intravenously) has been proved by Joachimovits. He showed that the repolarizing action of procaine is regularly followed by a reaction upon the basic tissues, by demonstrating that an initial disintegration of leukocytes in the capillary region points to the liberation of certain enzymes, followed by an increase in the number of monocytes, histiocytes, and mast cells, which are so important for the body’s spontaneous defenses. Some quaddles on top of the spleen (T8–T9) stimulate the organ of the immune system that is responsible for blood storage and result in direct improvement of the body’s defense mechanism. 10. Of crucial importance is the direct influence of procaine on the vital functions of the cell. When a nerve receives a stimulus, the bioelectrical cell potential is reduced, the selective permeability of the cell membrane is altered, the balance of sodium, potassium, and hydrogen ions is disturbed and cell metabolism, including cellular respiration, which is so important for maintaining the electrical potential, is inhibited. According to Fleckenstein, procaine also has a regulating function in these processes. It seals the cell membrane, protects it against electrostatic depolarization, and enables the partly discharged cell to recharge its physiological potential again. Procaine, according to Pischinger, intervenes as oxydizing principle in the process of cellular respiration, as a substance acting on the cell membrane and as inductor of the bioelectrical potential. With this new supply of energy to the basic tissues, previously inhibited autonomic functions are once again set in motion. In addition to reactivating the tissue and cell potential, the oxygen balance and other functions such as the mineral, water, leukocyte and ion balance etc., are also reactivated. As a result, the cell returns to being a functioning unit again. As eutonia is achieved at the autonomic and reflex stimulation levels, the stimulus threshold of the periphery is raised again. If all goes well, it will be raised to a level where pain remains subliminal and the organ is restored to a state of rest in which it can heal completely. 11. The redox system (reduction–oxidation system) is a metabolic chemical system that can absorb or desorb hydrogen depending whether its state is oxidized or reduced. The movement of electrons causes electrical potential: the electron conduction or redox potential. Its level indicates the reduction or oxidization potential of a redox system. The system with a positive charge oxidizes the system with a negative charge and the one with a negative charge reduces the positive one. With +290 mV (measurable with a platinum-calomel electrode), procaine has a high redox potential. Redox systems are important catalysts for the energy supply of cells. After dehydrogenation, they are responsible for the absorbtion and subsequent desorbtion of hydrogen to allow a gradual energy release, for example, during cell respiration. According to Warburg, depolarization of cytochrome c oxidase (“Warburg’s respiratory enzymes”) is the source of pathological processes because it deprives the cell of energy. In 1986, H. Lamers explained the healing potential of procaine with the fact that cytochrome c oxidase and procaine both have a redox potential of +290 mV. Procaine can repolarize and stabilize cytochrome c oxidase during depolarization, as long as the process has not turned autonomous. The flow of information and the regulation of metabolic processes in the basic autonomic system are restored. 12. In 1988, Professor Heine explains the neural-therapeutic effects of procaine as follows. Different from acupuncture, neural therapy uses the preferred pathways of somato-sensitive stimulation on the spinal cord level in two ways: through the injection point phenomenon and the local application of procaine. The injection point phenomenon produces an interneural pathway, i.e., preference of the affected somato-sensitive pathways with decrease or temporary elimination of only peripherally affected somato-sensitive and slower conducting viscero-sensitive pathways on the corresponding spinal cord level. This causes an interruption, particularly in the visceral feedback circuits, an “irritation pause,” located in the interference field and in the corresponding dermatome. Sufficient duration and some form of individual regulation capacity of the ground substance in the affected organ or area can induce regeneration of the ground substance and cellular functions (stimulation of individual self-healing abilities). This effect can spread autocatalytically, causing a systemic improvement of the basic regulation. (See Heine 1988, Perger 1987) Through the injection of procaine, neural therapy also extends the “irritation pause,” and by diffusion of the local anesthetic into the surrounding environment it covers a larger area of ground substance with terminal axons than does acupuncture. Neither the bond between procaine and axon membrane combined with the inhibition of membrane depolarization (Fleckenstein 1950), nor the redox potential charge of mitochondriae, is the primary cause for the increase of the “irritation pause.” Its primary cause is the bond between positively charged procaine molecules and acidic sugars of the ground substance components (glycosaminoglycane, proteoglycans, glycoproteins). This is supported by the following findings: if agar plates, used in microbiology, are colored with an aqueous solution of hyaluronic acid (0.1%) or chondroitin sulfate solution (0.1%) following procaine incubation with aqueous solution of toluidin blue (0.1%, pH 5.8), the metachromatic reaction is considerably lower compared with control tests. Isoelectric focusing shows the binding ability of procaine to polysaccharides. The bond between procaine and the above-mentioned 0.1% hyaluronic acid solution is the strongest when the ratio is 1:1. The bond between procaine and the chondroitin sulfate solution is the strongest when procaine is diluted with distilled water 1:100 000. The binding ability of procaine with sugar chains applies also to sugar components (primarily hyaluronate and heparan sulfate with terminal neuraminic acid) of the cell glycocalyx and to axons that end blindly in the ground substance. The anesthetic effect is produced through the neutralization of the charge between axon glycocalyx and axon interior, thus, the affected axon cannot be stimulated. This causes an interruption in the corresponding segmental feedback circuit: dermatome—muscles—viscera—peripheral nervous system—spinal cord—higher lever nerve centers—dermatome etc. If an interference field is located in the affected area, the interruption of feedback prevents the central representation of the noxious agent as pain. This is also a form of “irritation pause” that allows recovery of the ground substance. The extent of success depends on the precision with which the feedback circuit is defined by neural-therapeutic measures.” To put the matter in a nutshell: these local anesthetics, if correctly sited, produce not only a temporary nerve block, but create a complex regulating effect, and reactivate and regulate the functioning of the neurovegetative and basic autonomic systems. Their normalizing action on the regulating systems alone comprises an extensive range. In other words, sympathicotonic effects have been shown to occur, as well as parasympathicotonic ones, i.e., evidence has been provided that these products are able to restore equilibrium in the vitally important neurovegetative system regardless of its initial state or disequilibrium. On one occasion they can raise tonicity, on another they act as relaxants and reversants, as required. When used correctly, they are able to block excessive pathogenic reactions, which would otherwise initiate and establish pathological processes. Of the various theories on the manner in which procaine acts, let me here take Luzuy’s. This states that three factors act within one another: 1. The correlating balance is restored between the glands producing internal secretions. 2. The function of the diencephalon is regulated, especially its effect on capillary blood supply. 3. The harmful reflex arc is broken, including the antidrome effect, which manifests itself by massive histamine production and turns the sympathetic system into a pathological vasodilator. Despite its chemical relationship to a number of time-tested drugs and hormones, and the substantial number of useful properties that it has been proved to have, many questions remain open about procaine, which Reischauer called the “king of medicines.” Empirical medicine has discovered cures for which all the known theoretical and experimental foundations available to us are still unable to provide adequate explanations. In this connection, the following may be worth bearing in mind: for the neural-therapeutic effect as such, which, as time has revealed, is by no means limited to any single substance, there is no other satisfactory explanation in scientific terms other than my repolarization theory. The equalizing and regulating effect on the neurovegetative system that lasts far longer than the anesthetic effect itself and that occurs even at dosages that are not enough to produce complete anesthesia, is the essential factor and far more significant than the sum of all the pharmacological components. This is shown all the more clearly when we find that large amounts of procaine injected intramuscularly or intravenously may be completely ineffective whilst even a minute quantity, accurately placed in an interference field, can produce the far-reaching chain reaction that we witness time and again in the Huneke phenomenon. Eichholtz and Muschaweck reached the conclusion, based on wide-ranging investigations, that the effect of the local anesthetics used in neural therapy according to Huneke is perfectly reconcilable with orthodox scientific experimental medical doctrine. Originally, procaine was intended purely as a local anesthetic for use in surgery. But only a year after its discovery, G. Spiess, an ENT specialist in Frankfurt, published his observation that, apart from its anesthetic effect, procaine also developed therapeutic qualities and that it could be used to stop inflammation by infiltration around the affected area and thus allow this to heal more rapidly. Although this important detail was tested and fully confirmed at a number of hospitals, no attempt was made in Germany at that time to develop this line of investigation and these observations were allowed to be forgotten. The Pavlovian school in Russia paid greater attention to his work, but without recognizing the full extent of its therapeutic significance. This knowledge was recovered only when the Huneke brothers, in 1925, accidentally rediscovered the therapeutic effect of procaine and made it available to every physician when they built it up into their “therapeutic anesthesia.” They added the antidote caffeine to procaine, which, in larger quantities, can act as a convulsant, making it even safer for general use. It was soon found that the addition of caffeine not only reduced the toxic effect by half, but that it significantly increased the therapeutic effect at the same time. Nobody believed Huneke at the time. Sixty years later, Laska proved that an analgesic requires a 40% higher dose for the same pain-relieving effect if no caffeine is added. Caffeine acts as a vasodilator, notably in the region of the cerebral arteries, and of the coronary and renal vessels. It increases the permeability of the blood–CSF barrier and thus further reinforces the beneficial effect of procaine on the central nervous system. In 1928, Bayer Leverkusen put on the market and in the pharmacopeia this compound of 2% procaine and 1.42% caffeine as its effective agents in a sterile solution, under the registered name Impletol. The success of this product and the subsequent rapid spread of procaine therapy encouraged a number of other pharmaceutical firms to put “neural-therapeutic preparations” on the market. In some countries, Impletol is on the market in identical composition under different names. If any of these preparations listed are used, the dosages indicated in the instructions for use should generally be followed, although in our experience these are often too large, so that accidents due to unnecessarily high doses are conceivable. Some of the products also contain additives apart from caffeine, with its detoxicating effect on procaine, and these, in our view, are not necessary and do not constitute any improvement on the original. These additives are intended to produce reactions that have nothing whatever to do with the effective principles on which neural therapy is based and are, on the contrary, more likely to mask their effect. We therefore prefer pure procaine or Impletol, or the products corresponding to these, and to keep to the dosages stated in Parts II and III of this book. These were developed with the intention of further increasing the duration of the anesthetic effect. In the case of Depot-Impletol, resorption was retarded by the addition of Periston. This contains polyvinyl pyrrolidone (PVP) with the high molecular weight of 40 000, which, under certain reactive conditions, could produce foreign-body reactions. Other preparations have been produced with the addition of alcohol or an alcohol and oil additive. The manufacturers pointed out that the use of these depot preparations would produce irreversible degenerative changes in the nerve fibers and ganglionic cells, which would then lead to permanent blocking of the nerves. We regard surgical or chemical intervention that produces any permanent blockage of important nerve fibers to be a serious interference in the network of our vital nerves, which is bound ultimately to lead to consequences that we are totally unable to assess. We have therefore always refused to use depot preparations and have demanded their withdrawal. Their existence was due to outdated ideas foreign to the thinking on which neural therapy according to Huneke is based. We do not, of course, want to produce any long-term anesthesia. The ultimately decisive repolarizing thrust into the system can be achieved with the simple neural-therapeutic preparations without depot action, in a far less harmful manner. These destroy nothing, but if they are sited correctly, they restore order where it has been disturbed. No depot preparation can achieve more, even under the most favorable circumstances. Fortunately, all these depot preparations have now again disappeared from the market. 1. Plenosol is a mistletoe extract standardized to biological necrosis units. At intervals of 3/5/7 days, strictly intracutaneous quaddles of progressively increasing doses (from 0.1 mL of strength I, according to reaction, to 1 mL of strength II) are set around arthrotically and rheumatically altered joints (especially the knee). At the same time, any hyperalgetic points and nerve-exit points in the segment should also be sought out. Plenosol injected intracutaneously produces free histamine at the injection site, which, in turn, inhibits cholinesterase, because of which a protracted local acetylcholine effect is produced. Markedly diffuse paravascular aseptic inflammatory infiltrations occur, which persist for 3– 4 days. They penetrate in depth where they produce a persistently increased blood supply and a relaxation of the tissues. The stimulus produced by the inflammation is transmitted centripetally onward by the autonomic nerve-end fibers, switched over in the spinal ganglion and retransmitted centrifugally back to the segmental periphery where it increases the deeper blood supply. Whilst procaine combats inflammation, Plenosol produces it. If no Huneke phenomenon can be achieved in joint disorders and the joint fails to respond to periarticular procaine quaddles, there is reason to suspect a regulation paralysis (Pischinger). In such a case Plenosol quaddles may be indicated as an inflammatory counter-irritant therapy and achieve better results than can be obtained with local anesthetics. 2. Segmentan is a 1.29% aqueous isotonic solution of sodium bicarbonate and is particularly indicated for patients with procaine allergy, for intracutaneous quaddles, and intramuscular and intra-articular injections. 3. Sensiotin contains hypericin D5 and atropin sulfate D5 in isotonic NaCl solution. During treatment, extended exposure to strong lights should be avoided. Ampoules of 2 and 5 mL. In Seattle, in the United States, there is a pain clinic founded by the anesthetist Professor Bonica. Similar ones based on this example have been built all over the world. When anesthetists learned of the use of local anesthesia in segmental (neural) therapy, they used local anesthetics with amide structure that they were familiar with through surgery. They speak of “therapeutic local anesthesia” and tend generally to omit any due mention of the Huneke brothers as the originators of this therapy. The anesthetists like to use relatively large doses of the modern local anesthetics, as far as possible choosing local anesthetics with a long-lasting action, because they equate duration of the anesthesia with the therapeutic effect. They only consider the temporary blockage of nerve impulses to be the prerequisite for healing. We, on the other hand, believe that the repolarization and stabilization of depolarized cell membranes in disturbed areas with minimum doses of procaine, accurately placed for maximum effectiveness, is another aspect of neural-therapeutic phenomenon. Into the 1950s, procaine was the leading local anesthetic in surgery worldwide for all regional anesthesias and “nerve blocks.” For surgical purposes, the local anesthetics with amide structure, such as lidocaine, mepivacaine, or bupivacaine, have advantages over procaine and took over in the surgical area. For neural therapy according to Huneke, these advantages are irrelevant. The downside of the amide-structured local anesthetics shows, for example, in their longer detoxification time. Procaine, an ester of aromatic acids, is doubly and rapidly detoxified, for the most part immediately, by serum cholinesterase, by fermentation in the blood and tissues, and only a small part by conjugation of the liver. The amide-structured local anesthetics, on the other hand, are detoxified only in the liver. The substantial difference in detoxification shows clearly enough in the toxicity of these products, the comparative values being: procaine = 1, Scandicaine = 2, Carbostesin = 8! Care is therefore indicated where the patient suffers from liver damage, liver dysfunction, or is pregnant. Because of the rapid metabolism of the complete molecule of the product, procaine is less often accompanied by toxicity symptoms and, if such occur, they normally pass off more quickly than is the case with local anesthetics of the amide variety. Moreover, procaine poisoning has the advantage of presenting primarily as a respiratory depression, which is easier to control than the mainly cardiotoxic effect of lidocaine, which can quickly lead to ventricular fibrillation or asystole. Nor are the new local anesthetics able to seal off permeable capillaries (Hirsch). We cannot, therefore, conceive of any compelling reasons to stop using the time-tested product procaine, even though it is not supported by clamorous publicity, particularly since we know that procaine does not affect intercellular transport in the nerve fibers, whilst lidocaine (Xylocaine) inhibits this transport and hence probably also the nerve functions as such (Kreutzberg). We therefore use these other preparations (e.g. Xyloneural) only in proven cases of procaine intolerance, and then only in low concentrations and small quantities. We may summarize as follows. Any local anesthetic that does not contain a vasoconstrictor can be used for neural therapy according to Huneke. The least toxic preparation at the lowest concentration and in the smallest quantity adequate for the purpose is the best to use. It is also possible to achieve a neural-therapeutic effect, but to a lesser extent, without anesthetics, even by the intra- and subcutaneous injection of air. If one takes the needle alone and injects nothing at all, one is practicing a form of acupuncture, always provided the needle is correctly sited. The initial stimulus for the healing process can also be produced without a needle, by appropriate massage or any one of a large number of different forms of skin irritation. Every one of these therapeutic methods is intended to introduce outside energy into the tissue system, which will set off repolarizing effects in the basic autonomic system. The neural-therapeutic effect is thus the result of an unspecific reversant stimulus that is not limited to any given neural-therapeutic preparation, although such products apparently prepare the way for an even more far-reaching specific healing effect! Medicines as such are nothing at all if they are not used correctly. But if prescribed intelligently and after due consideration, they are the hands of the gods. Herophilus (fl. ca. 300 BC) The quantity of the neural-therapeutic preparation is always of secondary importance! The only crucial point is the correct site for the thrust into the neurovegetative system! The sick organism is, as it were, under stress when its own wonderful regulating systems are blocked and its spontaneous healing powers are therefore prevented from functioning. It always tends to restore normality. We call this normality health. If our injection strikes the correct spot, the effect is like that of cutting a tensed bowstring with a knife, by which the bow reassumes its original straightness. In the living organism, this means that the insertion of the acupuncturist’s needle or our injection at the correct site enables the body to pull out of its blocked state and allows the natural tendency towards equilibrium and normalization to regain its ascendancy once more and to become healing reality. The physician can only initiate the process. Nature (or whatever other label one wants to give it) heals: medicus curat, natura sanat. (Medicine cures, Nature heals.) Huneke’s neural therapy confirms the findings of acupuncture, which has continued to exist for more than 5000 years, simply because it works, because it helps the patient. He has freed acupuncture of its mystical accretions, made its essential elements clearly discernible, and simplified and complemented its complicated technique, making the art of the healing needle accessible to any physician. The healing stimulus given to the energy structure of the living organism by the thrust produced by the neural-therapeutic substance is, moreover, more comprehensive and more far-reaching than the needle on its own, because the local anesthetic introduces outside energy into the tissue system. The healing counter-stimulus should always be as small as possible. Arndt-Schulz formulated this point in the following effectiveness rule: “Weak stimuli rouse the vital processes, average stimuli promote them, strong ones hamper and the strongest prevent them totally.” Of equal importance to us, however, is the less well-known rider: “But it is an absolutely individual matter as to what stimulus will prove to have a weak, a strong, or the strongest effect.” The sick organism responds particularly readily to stimuli of all kinds. Even the weakest stimuli can produce extremely strong reactions. With procaine and its very wide tolerance, it fortunately happens only very rarely that a hypersensitive patient, or one who is greatly debilitated by long illness, will say that our treatment has affected him or her to such an extent that he or she has been obliged to stay in bed for a few days afterwards. In such cases, the patient’s stimulus threshold is so low that, for once, our stimulus becomes excessive for them. This fact should be recorded on their clinical record card. On the next occasion, procaine should be given to them only a drop or two at a time, at only a small number of injection sites, and the quantities increased only very slowly. Treatment intervals should be increased. The quantity of this healing stimulus that the patient needs and/or can tolerate, and the amount of procaine required to produce it (always the minimum possible!), vary with the individual patient and are largely a matter of the physician’s own “fingertip sensitivity.” As we have stated, these occurrences are so rare that there is no need to feel any anxiety about them. Fig. 3.1 Quick reference diagram for maximum quantities of lidocaine and procaine used in conduction anesthesia and local anesthesia, as recommended in Great Britain. The numbers refer to mL/kg of body weight. (Kelly DA. Use of local anesthetic drugs in hospital practice. BMJ 1983;286:1784. With increasing experience, one learns to use ever smaller quantities. Anyone who wants to practice neural therapy successfully needs once and for all to get rid of the idea that we can practice our healing anesthesia only if we flood the affected area with our neural-therapeutic preparation, in order to block the nerve paths. The terms “curative anesthesia,” “healing anesthesia,” “therapeutic anesthesia” and the like, used in the early days of neural therapy, were found misleading and have been dropped. As has been stated, it has been proved that in neural therapy the healing reactions are produced at concentrations of the pharmaceutical products used, which lie below those needed for anesthesia! Best of all is always the smallest possible stimulus that is just enough to produce a response from the neurovegetative system. More can all too easily be too much! If we speak of “stellate anesthesia,” for example, when using local anesthetics for neural therapy, it is not the same as a complete anesthesia for surgery! The healing stimulus, in the correct pinpoint position, produces a fundamental reversal, which affects the whole organism. This effect always persists far longer than the anesthetic action of the preparation as such. The secret of success, and one that does not simply drop into one’s lap, lies in the injection site, not in the quantity injected. A surgeon, for example, may “flood” an affected knee with 50 mL of procaine. We can achieve at least as good a result by distributing a mere 2 mL by means of five intracutaneous quaddles around the knee joint. The quantities given in the text refer to 1–2% procaine or 0.5–1% lidocaine solutions. They are intended strictly as indicative, and generally represent about the upper limits of the amounts needed. For test injections, 0.1–0.2 mL will often be enough! Where one is dealing with such small quantities, it is perfectly possible to carry out several test injections in a single session. We never give more than l mL intravenously, unless the injection is administered particularly slowly. Any dizziness that may occur following a rapid intravenous injection is of no account and wears off after a few minutes. Injection of cold solutions can be painful. In winter, the ampules should be held in one’s fist before use to bring them up to body temperature. 1. Procaine: The maximum dose for procaine given in the publications varies between 0.2 g (Swiss Pharmacopeia) for a single intravenous injection, to 5 g (Vishnevski) for infiltration anesthesia. Toxicity depends on the site of the injection, the concentration, and the time taken to metabolize the product. In accidents, patients are known to have survived 15 g, whilst in extremely rare cases fatal complications have been produced with far less than 1 mg. All theoretical maximum dosages are based on healthy adults weighing 70 kg. For children and patients in a reduced general state, dosages should be reduced by 30–50%. For procaine, the usual maximum dose is l g, i.e., 14 mg/kg body weight, equal to 100 mL of 1% solution. Since the toxicity of a local anesthetic increases as the square of its concentration, 100 mL of the 1% solution correspond to only 25 mL of 2% solution (100:2 × 2). The caffeine additive in the 2% Impletol preparation increases the procaine tolerance by 30–40%. In the areas of the head, neck, and genitals, a dose of 200 mg procaine within 2 hours should not be exceeded (Red List). Quantities such as these are far greater than anything we ever use even approximately in a single session! The very small quantities we use enable the patient to be fit for the road again after a waiting period of 20–30 minutes. There is no risk of habituation or addiction, even if procaine is given for lengthy periods. 2. Lidocaine: The maximum dosage is given as 200 mg, i.e., 2.9 mg/kg body weight. For Xylonest, it is 400 mg, for Mepivacaine 300 mg = 4.3 mg/kg body weight and 150 mg for Bupivacaine. The maximum dose for Xyloneural administered intramuscularly is about 20 mL. Because of the slower resorption as compared with procaine, road-fitness is achieved more slowly. All things are poisons and nothing is without poison. Only the dosage causes a thing not to be a poison. Paracelsus (1493–1541) At the correct dosages, i.e., with minimum doses used correctly, the risk in neural therapy of using local anesthetics is exceptionally small. “For therapeutic purposes only, 0.5–1% procaine solutions have proven effective, because the desired result can be achieved with minimal risk” (Killian). Complications are extremely rare. It has been estimated that the intravenous rate or complications for the large number of local anesthetics given daily all over the world is between 1:100 000 and 1:200 000. Most of these accidents result from overdoses. We are convinced that many reported procaine incidents can only be attributed to overdoses. Thus, 20 mL of procaine solution used for a “stellate ganglion block” can cause a serious situation simply because of the mechanical pressure exerted on the carotid artery, for which the preparation cannot be held responsible. In the highly sensitive cervical region, the tolerance limit for procaine and lidocaine can be exceeded if 5–10 mL are used, but with the 2 mL we use, this type of complication due to the carotid-sinus reflex cannot occur. Also, refer to page 287 on possible mistakes and complications in injections to the sympathetic chain. When one reads what are often self-important sensation-mongering reports on procaine damage, it is important to set its millionfold usefulness against the occasional and generally avoidable accident, and then decide whether to allow oneself to be irritated by them. Most of these incidents have been reported from dentistry. The majority of them are due not to the use of pure procaine, but of procaine with the addition of vasoconstrictors such as adrenalin or its derivatives. However, for the sake of comprehensiveness, let us now consider in detail the side-effects that may be produced and all the types of accident that could occur: Depending on the patient’s initial autonomic state, the quantity used and their tolerance, some patients will feel stimulated or excited after being treated; others comfortably relaxed. Dilation of the pupils, a faster pulse rate, dizziness, trembling, a higher blood pressure followed by a drop a little later, a sudden outbreak of sweating, and a sense of exhilaration akin to intoxication are reactions that pass quickly and prove to us the extent to which the sympathetic system and vagus have been involved and are responding. If Impletol is used, none of these should be attributed only to the caffeine component but should be regarded as the result of a general reaction and as a response to the healing stimulus. Euphoria that persists beyond half an hour cannot, of course, be explained in pharmacological terms. We take it as a welcome effect of the positive chain reaction that procaine is able to set in motion. It proves that we have been able to switch off a negative influence on the patient’s psyche. Because of this sense of euphoria produced in some patients, we must be careful to use considerable reservation in judging the success achieved when we make our assessment immediately following treatment. And the fact that the neurovegetative system as a whole tends to respond and react to any insertion of the needle does not absolve us from the need to seek and find the correct site for every one of our injections. When injecting local anesthetics from multiple dose vials, the required bacteriostatic additives (methylparaben, benzyl alcohol) may cause local irritations when injecting in the area of the spine, and toxic cerebral irritations, including headache, vertigo, vomiting, meningitis (Krauseneck), when injected intrathecally (into the spinal canal) in root diverticuli or root sheaths by mistake. This is the reason why ampoules are used when injecting neural therapeutics near the spine. They do not contain bacteriostatic additives or caffeine (as Impletol does). According to product information, multiple dose vials should only be used for 3 days and kept refrigerated after the first use. Procaine solutions with yellowish discoloration should be discarded. Procaine intolerance and allergy are a good deal less common than is generally assumed. Reischauer only allowed skin allergies following procaine and described all others as museum pieces. In 100 000 paravertebral anesthetics of the sympathetic system and the spinal segments, he did not observe a single allergy. In my first 20 years of extensive neural-therapeutic practice, involving what are by now innumerable patients, I witnessed procaine hypersensitivity on only three occasions. Two of these were women who tolerated subsequent treatment with lidocaine without further difficulty. One of them also proved allergic to iodine. Following an intravenous procaine injection and quaddles on the chest, there was circumscribed urticaria around each of the injection sites. Itchy reddening over a 2-year-old bone fracture was of particular interest. When this was tested with lidocaine, it proved to be an interference field responsible for her angina pectoris symptoms. We have often had the experience that following treatment, interference fields signal their presence at totally different sites. It is therefore extremely important that doctor and patient should look out for such signs. In the case of a 6-year-old boy, there was a severe allergic skin reaction after the initial treatment of a smallpox-vaccination scar, which was acting as an interference field producing progressive muscular dystrophy (Case History 29, Part II). Following a second injection, the reaction was considerably attenuated, and thereafter it did not recur, although the same preparation continued to be used. I learned of this allergic reaction only after the patient had been cured, or I should have changed the preparation used. But this taught me that procaine allergy, like any other, can also be due to an interference field. On the other hand, one is also bound to admit that during the last few years there seems to have been a general increase in procaine allergies. With the ever-increasing abuse of medicaments and the flood of new products being thrown constantly on the market by the pharmaceutical industry, the number of allergic patients has also increased. Thus, the frequently thoughtless use of procaine-penicillin against harmless colds or even for prophylactic purposes has doubtless increased, not only the number of penicillin allergies, but with it the number of iatrogenic procaine allergies. Substances have a longer-lasting effect when combined with procaine salts. For example, when added to penicillin, it slows down its decomposition and provides consistent effectiveness for hours. The slowing down of the resorption process is due to the poor solubility of procaine. Thus, procaine can be found as an additive for a large number of preparations, with the popular side-effect that intramuscular and intra-arterial injections are now pain-free. The therapeutic effect of procaine is credited to the preparation but not the allergic sensitization. Even the frequent use of sunburn creams with surface anesthetics can produce sensitivity to ester-based local anesthetics. P-aminobenzoic acid (PAB) occasionally causes an allergic sensitization attributable to the para-amino group. A para-group allergy can also occur with substances having a primary amino group in the para position in the benzol ring, such as sulfonamide, antihistamines, azo dyes, saccharin, ball-point ink etc. Part of this group is methylparaben, which is frequently added to local anesthetics as a preservative, particularly to multiple dose vials. About half the patients who are allergic to sulfonamides supposedly do not tolerate procaine well. A para-group allergy in the allergy record ought to warn us to be careful and suggests the performance of the tests outlined below. Hahn-Godeffroy calls “para-group allergy” a “mare’s nest.” He considers it merely a product of theoretical, structure-analytical thought that has found its way into pharmacological textbooks without sufficient clinical confirmation. Skin allergies do indeed occur, but a consistent sensitization with subsequent increased anaphylactic risk has not been reported since the 1950s. According to the textbooks, procaine allergy shows itself by reddening around the injection site, local edema, and, possibly, by a weeping eczema, which, in severe cases, may become generalized. In specialist literature, one can also find mention of allergic purpura and the formation of necroses. After administering the first drops of procaine, we observe every patient carefully to ascertain whether any symptoms of procaine intolerance appear. If in doubt, where the patient reports that he or she is allergic to Pyramidon and other procaine-related products, we set an intracutaneous test quaddle the day before starting treatment proper. If this test proves positive, i.e., if there is reddening and itching, we switch to 0.5% or 1% Xylocaine, 0.5% or 1% Scandicaine without adrenalin, or Segmentan, for his or her further treatment. The patient’s record card should be clearly marked to this effect, to prevent mistakes and accidents in any later treatment. If lidocaine is left for any time in contact with heavy metals, decomposition may occur. Filled syringes should not, therefore, be left lying around for any length of time, and after use, all metal parts should be thoroughly cleaned of any residue. A rapid test that is perfectly adequate for general practice and that furnishes the requisite information on the patient’s toleration of procaine is the procaine-conjunctival test. Before starting treatment, a drop of procaine, or whatever other neural-therapeutic preparation is to be used, is given into the conjunctival sac. If during the next few minutes the conjunctiva becomes markedly injected, this indicates that the patient may be hypersensitive. In addition, we also do an intracutaneous test, by setting an intracutaneous quaddle on the flexor aspect of the forearm and, at some distance from this, a control quaddle with a physiological saline solution. If the procaine quaddle and its surrounding area start to redden and itch during the next 10 to 15 minutes, an allergy must be assumed present and an amide-structured neural-therapeutic preparation, such as Xyloneural, should be used instead. The most dangerous complications occur when a large quantity of local anesthetic is accidentally given intravenously or intra-arterially (especially if it contains a vasoconstrictor!), since in such cases severe toxicity can occur immediately. Intoxication resulting from overdoses injected extravasally, on the other hand, will be slower and less dangerous. We also need to take into account that resorption is more rapid and thus occurs in larger quantities in areas where there is a good blood supply, e.g., in the region of head and neck. Moreover, procaine itself stimulates the blood supply. But let me emphasize once again: by using only the small quantities recommended and by aspirating before injection (especially when we are about to inject relatively large quantities), these risks can easily be avoided. With overdoses, there is the risk of procaine shock, which, in a reflex breakdown of vital central functions, can lead to death from acute shock, or the patient may suffer from convulsions or circulatory failure. A clear distinction should be made between hyperventilation tetany and procaine incidents as such. A procaine overdose acts as a convulsant toxin, and caffeine is used as an antidote. In Impletol, caffeine has already been added to the procaine in an ideal combination, because of which the procaine is largely already detoxified. In the small quantities normal in neural therapy according to Huneke, it can be used without hesitation. As has been stated, the maximum dose of 35 mL of procaine is never injected in a single session! A special warning must also be given against the use of adrenalin or supra-renin additives with procaine, especially for intravenous injections. These additives increase the toxicity of procaine by a factor of 10 (Keil, Rademacher). Paul examined 290 cases of poisoning through Novocaine (procaine). In 47 of 48 cases that resulted in the death of a patient, the cause was the application of procaine-adrenalin mixtures. Only one case resulted from overdose. Nor should patients who are under treatment with opiates (morphine, Dolantin, etc.) be treated with preparations containing procaine or, if they are, only with the greatest care, since opiates substantially reduce the tolerance ratio of procaine. In addition, the use of beta-receptor blocking agents (e.g., Aprinidin) together with local anesthetics should be avoided, since their effects can then become cumulative. According to Muschaweck, the addition of vasodilators, barbiturates, caffeine, or PAB reduce toxicity without considerable impact on the anesthetizing effects. Complications that can occur in injections to the → (T) sympathetic chain are dealt with in the description of the techniques for these injections in Chapter 8, p. 289. In all cases of mishaps, keep calm, do not panic! Act promptly and purposefully! Instruments and medicaments should always be within easy reach and ready for use. True mishaps and side-effects of neural therapy according to Huneke due to injection doses, types, and concentration are considerably lower than those caused by negligence of proper injection techniques. In 45 years of extensive practice of neural therapy I have not, with the exception of slight allergic skin reactions, ever witnessed any of the complications described below. However, for the sake of comprehensiveness, they must be included in a manual such as this. Everyone who practices neural therapy must know the risks (which are all avoidable) and must be prepared to deal with possible accidents. Vasomotor disturbances can occur due to anxiety, pain, and breath-holding, especially in autonomically stigmatized patients. These may lead one to fear a mishap, although, as a rule, they are absolutely harmless. Where the patient’s vascular regulation is labile, a massive drop in blood pressure may produce unconsciousness. Warning symptoms are pallor, slight nausea, a thin, racing pulse, shallow respiration, and sweating. Treatment Lie the patient flat, with legs raised. If he or she reacts to vocal stimuli, encourage deep breathing. If the doctor’s behavior is sufficiently assured and unflurried, this will generally induce a rapid recovery in the patient. If necessary, give oxygen. Circulatory stimulants such as Effortil are only rarely needed, and the same applies to valium, which, if given, should not exceed half an ampule. Verbal reassurance to the patient and anyone accompanying him or her will generally be all that is necessary. Severe states of collapse following irritation of the carotid sinus, produced by unskillful stellateganglion anesthesia, are true states of shock and must be treated exactly like anaphylactic shock. Make the patient hold their breath or place a hand over their mouth. Or have them breathe for 2–3 minutes into a 1–2-litre plastic or paper bag, which they should hold over their face, covering mouth and nose. At the same time talk to them quietly and, if necessary, demonstrate how to use the re-breathing bag. Table 3.2 below provides some countermeasures against allergic reactions. Table 3.3 provides countermeasures specifically against anaphylactic shock. Table 3.4 outlines the symptoms and procedures for treating overdose. If the patient is in severe shock, it may be difficult to find a peripheral vein for injection. The anonymous vein (brachiocephalic vein, sometimes also referred to as the subclavian vein), still remains available in an emergency. This is one of the largest venous trunks in the body. Its 20 mm lumen never collapses because it is fixed by interstitial tissue and the subclavian muscle. If the correct technique is used, the injection is practically without risk. No thrombus will form even if the needle is left in position for some time and after repeated injections. Technique: Turn the patient’s head to the side opposite the injection. Palpate the lower edge of the clavicle. At the osteochondral junction next to the sternum there is a flattening. The injection site for the 80 mm-long l mm needle is 10–15 mm laterally from this. To ensure that after passing the lower edge of the clavicle the needle will go in the right direction and to the correct depth, it should be pushed medially and slightly cranially towards the posterior surface of the head of the clavicle, aspirating all the way. The anonymous vein is reached after 30–60 mm. If the direction is wrong, one may accidentally pierce the subclavian artery, which is located dorsally from the anonymous vein, but this can be recognized easily by the bright red blood entering the syringe in spurts. After correcting the position of the needle and ensuring that it is definitely in the vein, proceed with the injection. The dome of the pleura is more posterior and is unlikely to be punctured, but should a minor pneumothorax occur, it will resorb by itself. In view of the small doses involved and its excellent tolerance, the procaine solution can be given without hesitation to patients with liver disease, arteriosclerosis, cardiac decompensation, myasthenia gravis, hormonal disturbances of all kinds, thymolymphatic state etc. For exceptionally sensitive and cachectic patients, minute quantities will often suffice. The whole of procaine therapy, using small dosages, and observing the few precautions explored in this manual, is absolutely free from risk and suitable for any patient. It is perfectly compatible with any other preparations and therapeutic methods (except those specifically mentioned) and can therefore be used at all times. In hypersensitive patients, where the psychological factor and the need to show off play a dominant part, one must not be irritated by reports of a worsening in their condition or of negative side-effects. The dosages for subsequent treatment may be reduced, stress being laid on the fact that neural therapy cannot ever harm the patient. A doctor must never let the patient take the reins out of his or her hands! When there is a change in the weather, a thunderstorm in the offing, or severe atmospheric disturbance, sensitive patients are more easily disturbed and irritable. They are more liable to collapse, and injection sites will tend to bleed longer. For these reasons, Japanese acupuncturists prefer, as far as possible, to treat patients only on days when the weather is less critical. It is also worth remembering that procaine (or, more precisely, the p-aminobenzoic acid and its ester) negates the bacteriostatic action of sulfonamides in vitro. Hence, the administration of sulfonamides side by side with procaine therapy is likely to be ineffective. If the administration of sulfonamides is indicated, procaine, which is resorbed after 30 minutes, or lidocaine, which is resorbed after 1–3 hours, is injected first and when the resorption time has passed, sulfonamide is administered. Long-term anticoagulants (such as Marcumar, Sintrom etc.) are a relative contraindication to procaine. Prophylaxis with these preparations against thrombosis and infarction is in practice effective only if the prothrombin value can be reduced to about 20–30%. But at this level the tendency to bleed and the possibility of severe bleeding following injury and deep injections are increased. In outpatient treatment, these ideal values are hardly ever achieved and generally remain within the 30–40% bracket. Cautious authors warn against intramuscular injections when the prothrombin value is below 70%, which, in fact, can be the case in liver or renal insufficiency even when anticoagulants are not being used. This value is certainly somewhat high, and a minimum around 45% is more reasonable and still acceptable. Test injections into scars and quaddling are still justifiable even where the clotting ability of the blood is reduced. But for purely forensic reasons, deeper intramuscular injections, anesthesia of the sciatic nerve, and especially injections in the vicinity of major blood vessels, should be avoided when the prothrombin value is below about 45–50%! If a patient on anticoagulants starts to bleed, vitamin Kl (Konakion) should be given as an antidote. As a rule, patients undergoing anticoagulant treatment are supposed to carry a supply of this with them. The constantly recurring theme in this book, of referring to possible mistakes, risks, and side-effects, is intended to provide all the safeguards the novice may need, but it should not be taken to suggest that these injections involve any kind of special hazard. On the other hand, it would be equally wrong of us to minimize the risks (which, after all, are always present wherever and whenever a physician has to act) or if we were to encourage fellow doctors to wield the syringe indiscriminately and to jab the needle without concern. This would be useless, and any injections that are not accurately pinpointed are bound to be of little or no avail. In fact, our problem is the reverse, since we need to infuse our colleagues with a little courage and, above all, to disperse their conditioned fear of injecting with the long needle. Time and again one reads of procaine accidents and even fatalities, particularly where the stellate ganglion is injected (one fatality in 10 000 injections!). A survey made by the Bayer pharmaceutical company found not a single fatality to have occurred as a result of using Impletol, in all the 65 years it has been in use. Mistakes made by individual practitioners should never be blamed on the method. Since surgeons occasionally give as much as 100–250 mL or more of procaine solution at a time, one ought to, when reading this kind of report, always ask what type of local anesthetic, concentration, and quantity, which additives and technique was used. Even though the risks are practically nil when the proper technique is used, every neural therapist ought nonetheless to have all the medicaments and appropriate equipment ready to hand for dealing promptly with possible emergencies. For the rest, any hesitation that places his or her own safety above that of his or her patient is unworthy of a doctor’s professional duty. Anyone who wants to help the sick cannot be afraid of personal sacrifice or shy away from the need to overcome whatever obstacles may be put in his or her path. And no doctor is ever too old to make new discoveries, to relearn, to learn a little more than he or she knew before! “We are not only responsible for what we do but also for what we don’t do” (Voltaire). There is nothing more difficult than making a start. For the beginner, I recommend that they learn, first of all, to decimate the hosts of those suffering from chronic headaches, by using the perfectly simple, straightforward intravenous injection and the injections under the scalp, and then to use these same injections for vertigo, insomnia, and post-concussion disturbances. By these, they will increase their experience and skills, and gain grateful patients, which will then give them the strength to continue further along this road. And when they have learned to help a patient with lumbago by means of a few well-placed quaddles, and with a few quaddles around the joint to reduce to a fraction the problems created by a painful knee, they will automatically discover other steps leading them to selective segmental treatment. At this point they will experience the pleasure of using a simple procaine injection to the trochanter major to turn someone who has been seriously ill with hip disease into a happy, healthy person once again. Suddenly, they will find that they have become intoxicated by procaine’s potential. And once they have succeeded in making a previously unresponsive disease disappear in front of their eyes with a Huneke phenomenon, by injecting a scar, the tonsils, or some other interference field, they will be a convert to this new art of healing to such an extent that the inevitable failures that come to every one of us will no longer be able to make them abandon the syringe forever. I myself stopped practicing neural therapy on two occasions, because things seemed to go less well than I had hoped; I could take the hurdle barring my way to knowing ‘where’ only at the third time of trying! Un peu moins de science, un peu plus d’art, messieurs! (A little less science, gentlemen, and a little more art!) Trousseau Before reaching for the syringe, it is essential to establish a thorough, purposeful clinical history and make an accurate examination in the conventional sense, supplemented by an extensive inspection and, particularly, palpation, in order to determine where our injections will be most effective. Following this (and after evaluating the reaction to the last treatment given to the patient) the plan of treatment for the present session is established and discussed with the patient. He or she is entitled to know what we are doing and why. We want to place our neural-therapeutic preparation with the minimum number of carefully thought-out injections where, as a result of our earlier examination, we expect to find disturbed and disturbing tissue. However, the technique for the injections must not be allowed to act as an obstacle between patient and doctor. With increasing experience and an intensive empathy with the patient, we can acquire the fine fingertip sensitivity that tells us in what neurovegetative state the patient happens to be. This then determines what we can expect him or her to accept on this occasion. We do not provide treatment for any given disorder in accordance with a blueprint; we are concerned with treating sick individuals who react differently from one another. Even the same patient does not always respond to the same stimulus in exactly the same way. The stimulus dosage must conform to the patient’s age, their constitution, the duration, and severity of their disorder, and to their general physical and psychological state. The intervals between treatments must also depend on the severity and duration of any positive and negative reactions produced by the treatment. As a rule of thumb we may take it that, in acute conditions, the quantity of procaine or lidocaine that can be administered and the number of injections that can be given will generally be greater and the intervals shorter. In chronic disorders, on the other hand, we generally start with smaller stimuli and approximately weekly intervals between treatments. The older the patient, the longer their disorder has lasted, and the more severe it is, the more their basic autonomic system is blocked, the smaller will be the quantities we administer, the smaller the number of injections, and the longer the intervals (up to 4 weeks between treatments). Before giving any injection, a neural therapist must have the following four points clearly in his or her mind: • site of injection; • direction of injection; • depth of injection; • what other factors must be taken into account. Pain should be kept to a minimum for the patient. My surgery assistants have standing instructions to hold the patient’s hand when I have to hurt him or her. This wordless help as a gesture of brotherly love is a good deal more effective as psychotherapy than a mass of words can express. During the injections, which I give in rapid succession, I talk to the patient in order to distract his or her attention. Even the instruction to breathe deeply and not to hold their breath is of help to them. From a psychological viewpoint it is also right, before giving any injection to painful areas (fingers, toes, perineum, mandibular nerve etc.) to warn the patient that this will now hurt a great deal. Thus prepared, he or she will bear the pain much better. Praise for showing courage is always welcome. A little joke is always far better than a scientific lecture. There was always an air of jollity in F. Huneke’s practice. I have adopted this as a desirable objective worthy of emulation, although, despite this, one can still take one’s work very seriously indeed. As we have seen, segmental therapy encompasses all our efforts at the site and within the relevant segment of the disorder. If, for example, in a mainly right-sided migraine, I inject l mL of procaine into the right antecubital vein, bilaterally under the scalp, to the hyperalgetic spot confirmed by palpation at the exit point of the right supraorbital nerve and another to that of the right suboccipital nerve, then this carefully selective polypragmatic approach consisting of five injections constitutes a single segmental treatment. From segmental therapy, we expect only a substantial improvement. Even if all pain and symptoms disappear instantly immediately after segmental treatment, we do not describe this as a lightning reaction, as a Huneke phenomenon. Before further treatment, we must first await the patient’s particular reaction to the previous treatment we have given them. If a treatment series is repeated too soon, it may result in worsening the patient’s condition instead of improving it. In such cases, the stimulus strikes a (positively) altered organism, which may respond differently, perhaps negatively, to the new stimulus. Let sleeping dogs lie! Speransky taught that “if the effect of the treatment is positive immediately, then the interval should be increased before repeating the treatment or giving further treatment.” “More helps more!” does not apply here, neither with regard to the number of injections nor to the number of treatments. An excess of stimuli can only harm. Often, the body responds either not at all to a barrage of too many injections, accounting it as an excessive stimulus, or its response may be negative. One must not, therefore, give in to pressure by the patient in the sense of “another one here and another one there!” Only if the disorder recurs should the treatment by which a temporary improvement was achieved be repeated. It should be carried out at the same site and in the same manner as before. Such repeated segmental treatment must then increase in effect until a complete cure has been achieved. If the first treatment in the segment has produced no improvement or if the improvement is only temporary and its effect cannot be satisfactorily increased on repetition, further segmental treatment at the same site is pointless and should be abandoned. It is possible in the course of treatment, after one or more interference fields have been removed, that segmental treatment shows improved results. In that case, interference field and segment can be treated together. Thus, if in our case of migraine the treatment described above has failed and injections to the Gasserian (otic), stellate, pterygopalatine and ciliary ganglia, to the temporal, vertebral, and carotid arteries, an infiltration to the posterior third of the nasal conchae, and a nasal spray have also proved ineffective, all further efforts in the segment are pointless. The segmental treatment of migraine comprises all these measures. Any single one of them may be adequate by itself for the patient in question and may lead to success, possibly after repeating the treatment a number of times. There are no two identical diseases, since there are no two identical human beings. Hence, everything stated in this book can be regarded only as a series of tips and suggestions outlining some of the potential available to us through neural therapy. Only someone fully familiar with all the possible injections and who knows how to make intelligent use of them is a practitioner of neural therapy according to Huneke. No one who is not familiar with the method should presume to sit in judgment over it. And now, what happens next to our migraine? The following basic rule cannot be repeated often enough: if our conventional methods have failed and segmental therapy has not taken us any further forward, our next task is always to look for the interference field! In the case of our migraine patient we find the following in her history. As a child, she suffered from diphtheria, scarlet fever, and frequent sore throats. First menstruation only at the age of 16, dysmenorrhea with nausea and vomiting. Migraine started during first pregnancy, forceps delivery with episiotomy; later two miscarriages, one febrile. Carbuncle scar on back, barely visible scars on inside of both first metatarsophalangeal joints following hallux valgus surgery. Several teeth with dental crowns and pivot teeth. Where should we expect to find an interference field? The tonsils could be the culprits, the pelvic region is under considerable suspicion, and we know that any scar and any devitalized tooth can be an interference field. Further, we also need always to bear in mind Huneke’s thesis and take it literally: any disorder may be caused by an interference field; any point of the body may become an interference field! We therefore first test the tonsils at the upper and lower tonsillar poles, since the tonsils are the most frequently encountered interference field of all and can be tested so easily. The patient states that her headache remains unchanged. We mark her record sheet: Tonsils 0. After the negative test result any further injection to the tonsils is pointless. This is followed by an injection into the pelvic region. The patient tells us: “All of a sudden my head feels so light, all the pain is disappearing like morning mist when the sun comes out.” After waiting a few minutes, she tells us happily that all her symptoms have completely disappeared. We mark her record: Pelvic region +. As instructed, she comes back 3 weeks later because her headache has returned. She reports that in the meantime she has been feeling very well, that she can now sleep without tablets, and that the unpleasant vaginal pruritus that she did not mention before out of embarrassment has gone. She also reports that she no longer has pins and needles in her hands at night. Although her headache tried once or twice to come back, it has been a fully-fledged migraine only since yesterday. From this we learn that a single interference field may act as trigger for and keep several disorders going at the same time. Obviously, on this occasion we treat only the pelvic region. Again, the symptoms disappear at once. We mark her record: Pelvic region ++. We dismiss the patient and tell her to come again if pain recurs. Three months later, further treatment becomes necessary. The woman has meanwhile blossomed out and is more than happy to have found relief after so many years of suffering. She tells us that she has been completely free from pain until it reappeared after a strenuous spring-cleaning. Thus, the second treatment remained effective for longer than the first that helped her 100%, and that had lasted longer than the required minimum period of 20 hours. All the conditions for a lightning reaction as established by Huneke have now been met and I can mark her record with three crosses: Pelvic region +++ = Huneke phenomenon. Unfortunately, matters do not always run as smoothly as that. Often, the information provided by the patient about his or her clinical history and the effects of the injections is so unreliable that it can be used only with considerable reservation and care. Frequently, there is the slow, wearisome business of searching, discarding, groping towards a conclusion. There is no rule about whether or where one ought first to look for an interference field or start with segmental treatment. Where the patient is in acute pain, the segmental approach as a first step is always right. It is also, of course, possible to combine both methods at a single session, first giving one to three test injections and then, if these produce no results, switching to segmental treatment. It is most important to keep a complete and accurate record of all injections and in the order they are given! Failure to do this means that one is bound to lose control and will not know the next time what has already been done and what should be done next. Anyone who subjects a patient to a barrage of injections, in the fond hope that one shot is bound to hit the target, should at least have the decency not to call this type of quackery neural therapy. Our objective is not to give merely momentary pain relief and produce a short-term improvement in the patient’s condition. To restore the function as far as this is still possible must be at the core of our treatment. Whilst our treatment plan must always be flexible and adaptable according to the reactions it produces, it must never be uncontrolled. Hence, any change found on palpation or in the mobility of a joint must be marked in addition to what the patient tells us. We mark every subjective and objective improvement with a +. In segmental therapy, this is enough for further injections to the same site (s). In looking for an interference field, it may be worthwhile repeating the treatment and, if this still fails to produce any adequate results, to subject the neighborhood to a more thorough examination. There is such a thing as a neighborhood reaction, an incomplete response to an injection given near an interference field. For example, the tonsil test produces almost 80% freedom from symptoms for 12 hours (++), injection to the adjacent wisdom tooth 3 days 100% (+). Repeated treatment shows with increasing success after each successive injection that the wisdom tooth is the interference field in question: ++, +++. If the patient states that his or her disorder has worsened as a result of the treatment we have given, we need to wait and find out if this is a strong reaction for 2 to 3 days that disappears, after which the condition improves. Treatment helps when the injection is sited correctly, but if the site is wrong it will not do any harm. True worsening of the original complaint after segmental treatment is a strong indicator for an interference field instead of a problem in the segment. No doubt after an injection to the tonsils the patient feels as though he or she is starting a sore throat, and an injection into the periosteum may produce a sleepless night due to pain. But a severe reaction should not be regarded as being the same as a worsening of one’s condition, and the neural therapist should on no account let it induce him or her to break off the injections. A severe reaction, for example, after an injection into the periosteum or the pelvic region, normally persists for only 2 or 3 days, and when it begins to wear off the old symptoms normally disappear with it. In our ledger, initial “worsening” followed by substantial improvement or freedom from symptoms is shown as follows: 00– (++) or +. For instance, if a correctly executed tonsil test triggers (in exceptionally rare cases only!) a renewed attack of polyarthritis, this is known as an “inverted Huneke phenomenon.” It proves that there is a causal relationship between tonsils and polyarthritis, but it also shows that the organism is reacting abnormally, a state of affairs that first needs to be altered by reversant measures, before further treatment of the tonsils is undertaken. Negative reactions are always extremely rare. It is always worth waiting to see whether what starts out as a negative reaction will not ultimately turn into a positive response. Such an initial worsening is not unknown to us, for example, in homeopathy. It is a sound principle that no physician should ever allow his or her patient to take control. A doctor who has once shown him or herself to be uncertain is someone in whom the patient can no longer place his or her full confidence. The beginner may easily become confused by the wide range of possible injections available, some of which will doubtless be new to them. Obviously, we always start with the simplest injections that might be adequate for the case before us. In addition to the intravenous injection, which we use as the basic treatment, especially for all disorders affecting the upper half of the body, we normally use the proven quaddle therapy over painful, tender, itchy, or otherwise pathologically altered parts of the body. Often enough, the stimulus effect of these is enough to stop relatively minor pathological processes, and to arrest others in their initial stages and reverse them. If they do not suffice, we look for deeper-lying tissue within the segment that may show changes: subcutaneous tissue, muscles, tendons, ligaments, bones, joints, pleura, peritoneum. The next step takes us to the afferent nerves and arteries. The most invasive intervention is the direct injection into the sympathetic system, by going for the sympathetic chain and its ganglia. But normally we always keep these injections to the last; never use a sledgehammer to crack a nut! The basic rule must be: always use the simplest means first, with small amounts of procaine and the minimum number of injections, but these must be accurately sited. The smallest healing stimulus that is only just enough to set off the change is the best and least risky. We can only test suspect sites with procaine, and these we need first to seek out. We know that the effect of our individual injections can be proved objectively. But during our daily surgery sessions we need to depend on eliciting the reactions to our previous injections by questioning the patient. These questions have to be formulated precisely and purposefully, and we must insist on clear answers: Did they feel completely better, a little better, unchanged or worse after our last treatment? For how long was freedom from symptoms or the improvement maintained? Were the pain or the attacks less frequent or less severe? Was it possible to control them more easily with medication than before? Did a scar, a tooth, an old injury, or an organ make its presence felt that day or the next, i.e., has any pain appeared in any untreated part of the body? Everything should be recorded, including the weather (thunder-storm, weather change etc.) and personal details that can produce an additional strain on the autonomic system (death, divorce, period etc.). We therefore need to take the time to talk to our patient and to let them talk to us. We should observe any gestures that they make, since they will often unconsciously show us by these where we ought to make a start. The patient will come to us accustomed to telling their doctor only a few of their symptoms before a quick diagnosis is made and a prescription written out. With us, however, they will first have to learn that they are not only allowed but expected to talk, and that we are dependent on their cooperation. They will gratefully note that we are prepared to give them all the time they need, that we take their comments seriously, and that, before reaching for the syringe, we examine and palpate them thoroughly. Every attempt to do a “2-minute-add-on-neural-therapy” is half-hearted and doomed to fail. The real art consists in digging the right segmental possibilities out of the bewildering choice available and, if necessary, in combining them intelligently. What may simply seem to be a form of polypragmatism needs to be properly considered and purposeful, because it must be adapted as far as possible to the complex causality of the pathological processes we are confronted with, always provided, of course, that our theoretical ideas conform to the factual situation. In acute cases, the intervals between treatments may be anything from 1 to several days, whilst in chronic cases, as a rule, they are initially about a week. These intervals have to be adjusted individually and increased once we have discovered which injections are effective and how long the patient has remained free from symptoms. Ultimately, it is the patient who determines when further treatment is required. They are instructed to let us know immediately if the old symptoms recur after a symptom-free period or become more severe. The longer they wait the more treatments will be required. With chronic patients whose general condition is reduced and whose stimulus threshold is low, we use small quantities, few injections, and longer intervals of up to 4 weeks. Our therapy with local anesthetics can in principle be combined with other forms of therapy, such as manipulative treatment, acupuncture, homeopathy, physical medicine, and medication (with the exceptions as stated). To do this in every case and at once would, in my view, be mistaken, since to proceed in this way would make it impossible to recognize and identify clearly the causative processes at work, and the specific effects of any particular treatment given and of the injection site. One fact is certain—that other forms of therapy, including those based on medicaments, can often succeed only once an interference field has been eliminated. Interference fields can allow the control circuits to deviate to such an extent in the sense of positive feedback that normally successful methods of treatment must fail, regardless of whether they are based on medication, manipulative medicine, or whatever. Correctly sited local anesthetics can eliminate such interference fields and re-normalize the deviant control circuits to the point where they again respond normally. Only some of the injection techniques used in neural therapy were developed by the Huneke brothers and their pupils. Others, such as the “blocks” of sympathetic ganglia, paravertebral injections to the trunk of segmental nerves, and peridural anesthesia, have been taken over from anesthesiology, orthopedia, and neurosurgery. We consider them part of neural therapy according to Huneke, like all methods that use local anesthetics for therapeutic purposes (and not for local anesthesia!). Many of the techniques described here are used very frequently indeed, whilst others are employed only rarely. In order to indicate to those who are new to neural therapy the techniques they should master first and which they ought to concern themselves with particularly intensively, a star system has been used to mark the various injections: * of minor importance; ** important; *** extremely important. It should be emphasized that these are not qualitative judgments! It may be the injection that has a lower classification that is alone capable of yielding results. The quantities given in milliliters refer in each case to a suitable neural-therapeutic preparation of l–2% procaine or 0.5–1% lidocaine solution without the addition of vasoconstrictors such as adrenalin, epinephrine, Suprarenin etc. If a number of different products are used during a single session, the toxicity of the individual preparations and the maximum permissible doses stated must, of course, be observed! Adenoids → (T) tonsils. After the World War I the successes achieved by surgery of the sympathetic chain (Leriche) attracted considerable attention. Surgical removal of the nerve reticulum surrounding the arteries produced vasodilation in the periphery of the area supplied by them. In a number of cases, such surgery was able to deal successfully with extremely severe circulatory disturbances with threatening necrosis. But in the end the surgeons that specialized in work on the sympathetic chain discovered for themselves that para-arterial injections of procaine produced much the same effect as sympathectomy, as a result of which they abandoned surgery in most cases in favor of injection, described by Leriche as the “surgeon’s bloodless knife.” The procaine injections also have the advantage of being repeatable as often as necessary and cause no permanent damage to the nervous system, because they neither have a trigger effect in Speransky’s sense, nor do they leave an interference field behind. The effectiveness of para-arterial procaine or lidocaine injections can only be explained if we accept that the neural-therapeutic preparation acts as a regulating stimulus on the sympathetic periarterial tissue reticulum and thus restores the blood supply and other dysregulations to normality. Their ultimate therapeutic effect is similar to that of injections to the → (T) nerves (afferent). Fig. 3.2 Aspiration in two directions before injections in the head and neck area. 1. The tip of the needle is positioned close to the interior wall of the artery. Blood is not drawn into the syringe, in spite of the intravasal position of the needle, because the internal artery wall is sucked against the opening of the needle. 2. If injection takes place at this point, the arterial wall is pushed away and the local anesthetic is injected intra-arterially, in spite of a negative aspiration test! 3. If the needle is rotated 180 and a second aspiration takes place, blood will be drawn into the syringe. This reveals the intravasal position of the needle and unintended intra-arterial injection is prevented. 4. The small quantities used in neural therapy (low concentration solutions without additives), are unlikely to cause serious complications, even in the case of intra-arterial injection. An additional safety measure in the area of the head and neck is the injection of a small fraction of the intended amount near the carotid artery and the vertebral artery. If the patient does not display any adverse reactions after a short while, the entire quantity can be injected. Care must be taken to avoid injections into arteries supplying blood to the brain and leading cranially from the heart, since such injections can produce dangerous complications. Always aspirate to make certain (see Fig. 3.2)! This precaution must continue to be recommended in practice, although recent clinical experience has shown that the risk involved in an accidental procaine injection into the carotid artery is not as great as was originally assumed. Before Huneke, pharmacologists prophesied fatalities following intravenous injections of procaine; they then warned against injections into the carotid; now they are equally vocal in their objections to the → (T) cisternal injection according to Reid. In the Soviet Union, procaine injections directly into the carotid have been used for years in order to produce a direct therapeutic effect on the brain. Doronin injected 10 mL of a 0.25% procaine solution directly into the carotid artery of 150 patients with skull/brain injuries. Only four patients died after this injection, and these had already been in a terminal state when admitted. In animal experiments it has been found that the intra-arterial procaine injection into the carotid seals off the focus of traumatic brain damage so that pathological reflex effects on undamaged parts of the brain are prevented and functionally blocked brain cells are reactivated. The effect of this is positive not only as regards the brain itself but also on the organism as a whole. Nambiar gave 400 hemiplegic patients 10 mL of a 1% procaine solution into the carotid and reported good-to-satisfactory results, without a single fatality. The theory-based fear that damage will occur to the vascular walls if an artery is perforated repeatedly is not borne out by fact. In a total of 6000 patients suffering from vascular disease, Loose reported that he had in some cases injected 100 times into the same site in an artery without witnessing any serious incidents. Even fairly severely sclerosed arteries are still sufficiently elastic to close the injection canal. So, for example, in the case of diabetic gangrene, it is possible to inject into the femoral artery once or twice a day. In advanced obliterant arteriopathy, intra-arterial injections form the most effective conservative therapy and do not present any special risk (Alexander). From the effects of intravascular injections, both intra-arterial and intravenous, we can conclude that the local anesthetic injected is capable of eliminating autonomic control defects involving the blood vessels and the areas supplied by them. Injections into the following arteries will be discussed over the next few pages: 1. temporal artery, p. 290; 2. carotid artery, p. 290; 3. aortic plexus, p. 291; 4. vertebral artery, p. 292; 5. subclavian artery, p. 293; 6. brachial artery, p. 293; 7. femoral artery, p. 293; 8. posterior tibial artery, p. 294. 1. Temporal Artery* The superficial temporal artery is a terminal branch of the external carotid artery. It can be palpated easily as it pulsates subcutaneously anterior to the ear. For temporal arteritis and migraine, Leriche recommended infiltration around the temporal, facial, and occipital arteries. We also infiltrate around the artery with 2 mL in the case of headache, impaired cerebral blood flow, and conditions following apoplexia. An intra-arterial injection into the temporal artery by mistake is generally harmless, since this artery supplies only the periphery and does not lead to the brain. However, it can become dangerous if larger amounts of a local anesthetic are injected with high plunger pressure. A backup into the area of the internal carotid artery could transport procaine into that artery, where it could cause complications. Accounts of this occurrence can be found in the literature. Under the same circumstances, blockage of the internal carotid artery can cause the blood to return into the skull through existing anastomoses from the terminal branches of the superficial temporal and facial artery to the terminal branches of the supraorbital artery. In the head and neck area, particularly when we know of a blockage of the internal carotid artery, we will always inject slowly, after aspiration, and only small amounts of procaine to (not into) the temporal artery. 2. Carotid Artery (Carotid Body)* Para-arterial injections only! Alternative terminology Anesthesia of the carotid body, carotid sinus block. Anatomy and function The carotid body lies in the bifurcation of the common carotid artery. It belongs to the parasympathetic paraganglia. These are organ-like formations, which act as sensors for the control of vasodilation and the reduction of blood pressure (pressoreceptors), which function with the regulator in the medulla oblongata. In addition, it also possesses chemoreceptors, i.e., sensors that react to chemical stimuli, which keep the blood’s oxygen content constant. When the carbon dioxide level of the blood rises, the regulating respiratory center is stimulated to reduce the carbon dioxide level again by increasing the respiratory rate. It is thus an important control center for circulation, oxygen supply, and respiration. Three important nerve branches start here, one to the glossopharyngeal nerve, another to the vagus nerve, and the third to the upper cervical ganglion. Pressure due to strangling or a blow to the point where the common carotid divides can produce the carotid-sinus reflex, resulting in vasodilation, especially in the lower abdomen, and in slowing the pulse, and is even capable of causing cardiac arrest. Indications These are given by the carotid body’s influence on cardiac, vasomotor, and cerebral regulatory control centers. This explains, for example, the effectiveness of this injection in the prophylaxis and therapy of traumatic and post-operative shock with vasomotor collapse. This success is possible even when other therapeutic measures fail. In intestinal infarction it restores the balance in the diencephalic center of capillary regulation. It should also be tried in cerebral disturbances, lung disorders, disturbances in the conduction system of the heart, Ménière disease, therapy-resistant forms of headache, and in eye disorders where access is difficult, such as glaucoma, ophthalmic herpes zoster, painful disorders of the cornea, and others that have failed to respond to other attempts at treatment. Carotodynia → neuralgia. Materials Size 1 × 40 mm-long needle. Quantity 1 mL. Technique The injection is not difficult, but it must be carried out with the appropriate care. The patient lies on his or her back and turns the head slightly towards the side opposite to the injection site. The bifurcation of the carotid is at the level of the upper edge of the thyroid cartilage, where it can be readily identified by its easily palpable pulse. We fix the artery by pressure (not too hard!) and insert the needle vertically (without the syringe) so that when the needle is released it vibrates in time with the pulse rhythm. The assistant now carefully attaches the syringe and aspirates to check that it is still extravasal. If no blood is aspirated, we very slowly inject only about l mL of the solution immediately next to the carotid artery. Rapid injection or an excessive quantity, such as the 10 mL used by others, could be fatal! The injection must never be given bilaterally. After the injection, check the patient’s blood pressure. If the blood pressure decreases considerably and a carotid sinus reflex, caused by cranial ischemia, occurs, immediately place the patient into the Trendelenburg’s position (head slightly lowered, pelvis and legs elevated), which will normalize the blood pressure quickly and without medication (Koster, Kasman). 3. Aortic Plexus* Para-arterial injections only! In neural therapy according to Huneke, the injection to “Arnulf’s pre-aortic plexus” is hardly ever used. In angina pectoris, as a rule, we manage perfectly well with segmental therapy using appropriate quaddles and anesthesia of the stellate ganglion. According to Leriche, the injection to the stellate ganglion has about the same effect as that to the aortic plexus. But since the latter also forms part of the therapies using local anesthetics, it must be mentioned here. Anatomy This plexus innervates the coronary vessels. It is located in front of the aortic arch and consists of nerve fibers of the cervical sympathetic system and of the vagus. By blocking its action, the coronary vessels are dilated. Indications Angina pectoris, precordial pain in aortitis, myocardial infarct. Materials 0.9 mm 100–120 mm-long needle. Quantity In the original method, 20 mL of 1% procaine solution was used, but 5 mL should do equally well. Technique In the technique described by Arnulf in 1940, the patient is in a half-sitting posture, with his or her head laid back and turned slightly to the right. The needle is inserted through a quaddle 20 mm above and 20 mm laterally from the left sternoclavicular joint. It is guided behind the sternum, parallel with it and about 70–80 mm obliquely down toward the interior (approximately toward the center of the sternum). It is now in front of the aortic arch, immediately next to the point where the aorta leaves the heart. The aortic pulse is transmitted to the needle. The needle is not advanced beyond this point, and the anesthetic is injected slowly after aspiration proves negative. When 20 mL are injected (but not with 5 mL), the patient feels a heaviness behind the sternum, but this disappears rapidly. Any aphonia due to anesthesia of the recurrent nerve disappears as the anesthetic effect wears off. If this method is used there is no risk of injury to pleura, brachiocephalic (anonymous) vein, or aorta. If, exceptionally, the anonyma should be injured, apply pressure with a finger or pad in the hollow above and behind the clavicle. If the needle accidentally punctures the aorta, this does not matter (as in aortography). If necessary, the injection may be repeated three to four times weekly. Its effect will increase until it becomes permanent. Para-arterial injections only! Anatomy The vertebral artery runs cranially through the transverse foramina from the sixth cervical vertebra. Between axis and atlas it turns aside to the transverse foramen of the atlas, which lies further laterally. Above the atlas it again runs medially in a sulcus and penetrates the atlanto-occipital membrane and the dura mater. It then passes through the foramen magnum into the cranial cavity where it unites with the vertebral artery of the other side and becomes the basilar artery. The sympathetic supply of this artery is provided by the vertebral nerve of the stellate ganglion and by fibers of the upper cervical ganglion. The cranial nerves IX and X provide it with its parasympathetic fibers. The regions supplied by this artery are: spinal cord, pons, labyrinth, cerebellum, occipital lobe, basal portion of temporal lobe, midbrain. Indications Vertebral-artery syndrome, sudden syncope with brief loss of consciousness and crumpling of the legs, therapy-resistant forms of migraine and cervical migraine, positional vertigo with or without nausea; nausea, vomiting, and tinnitus, Ménière, upper cervical syndrome, conditions following → whiplash syndrome and other trauma in the area of the cervical spine, shoulder pains, ataxic locomotor and static disturbances, nystagmus, visual disturbances with loss of visual field; hazy, cloudy, or distorted vision. Scheffel took the view that anesthesia of the vertebral artery could be used instead of anesthesia of the stellate ganglion and listed further indications: multiple sclerosis, post-apoplectic state, facial paresis, hiccoughs, tinnitus, glaucoma, and bronchial asthma. Materials 40 mm-long needle. Quantity 1–2 mL. Technique a. The neural therapist Auch described an injection technique to the posterocaudal surface of the lateral part of the atlas arch. With this, it is possible to reach the vertical and horizontal portions of the vertebral artery, and the suboccipital, second cervical and greater occipital nerves. The patient sits with head bent slightly forward. The injection must not be given unless the site to be infiltrated, i.e., the lateral side of the atlas arch and its posterocaudal surface, can be palpated with absolute certainty. The point of entry is about half to one fingers’ breadth below and about one to two fingers’ breadths medially of the mastoid process, generally at the medial edge of the sternocleidomastoid muscle, but this muscle often has to be pushed slightly aside in a lateral direction. The direction of the needle is slightly craniomedial. The injection depth is about 10–20 mm and the local anesthetic is injected after a negative aspiration test. The patient should remain recumbent for 15–20 minutes after the injection. (see Figs. 3.3, 3.4). Fig. 3.3 Injection to the vertebral artery. Fig. 3.4 The injection site on the lower lateral arch of the atlas vertebra. b. Scheffel goes in a little further caudally, about two to three fingers’ breadths below the mastoid where the lateral process of the axis (epistropheus) can be felt at the posterior edge of the sternocleidomastoid muscle. After aspiration, inject 2–3 mL at a depth of about 15–20 mm at the dorsal portion of the epistropheus. Thus, both these authors go into the space between atlas and axis, Auch further cranially to the lower surface of the lateral atlas arch, Scheffel to the upper edge of the axis. In their end effect these two methods are identical. c. A third possible method of reaching the sympathetic reticulum surrounding the vertebral artery is to place a depot of 2 mL in front of the artery’s entry point into the vertebral canal in the lateral process of the (fifth or) sixth cervical vertebra. This can be easily found by palpation and is reached without difficulty from the front or side. d. Post-ganglionic fibers travel directly from the stellate ganglion (cervicothoracicum) to the adventitial plexus of the subclavian artery. The vertebral artery can be affected through anesthesia of the stellate ganglion or perivasal injections around the subclavian artery. This way, the areas of the posterior skull sulcus and the inner ear can also be treated. Possible complications According to Schmitt, accidental injection into the vertebral artery produces lightning-like flashing sensations in the homolateral eye, and tinnitus and hammering in the homolateral ear. A slightly comatose state disappeared after about a minute without subsequent symptoms. 5. Subclavian Artery* Para-arterial injections only! Indications and technique are obvious from what has been stated above and from the description of the injection to the brachial plexus (see → (T) nerves, afferent). Periarterial nerve plexuses travel from the stellate ganglion to the subclavian artery at the same location where the vertebral artery branches off. This is why Dittmar recommended the perivasal infiltration of the subclavian artery instead of the injection to the stellate ganglion for improving the peripheral blood supply and reducing hypertonicity of the body’s musculature in the region supplied by this vessel, particularly in stenocardia and in functional and organic cerebral circulatory disturbances. Because of the nearness of the cervical pleura, the needle must on no account penetrate beyond 15 mm! See also → scalene syndrome. 6. Brachial Artery* Intra- and Para-arterial Injections Indications Circulatory disturbances, support for the healing of fractures, injuries, burns, or congelation of the upper extremities and post-traumatic pain, nocturnal arm dysesthesias, arm plexus neuritis, Sudeck’s dystrophy, arterial emboly, acroparesthesias, causalgias, amputation/phantom pain, epicondylitis, styloiditis, tendovaginitis. If it is not possible to give an intravenous injection in small children, e.g., in otitis media, we inject to the brachial artery. Materials Size 2 needle. Quantity 1 mL. Technique The brachial artery is found by palpation above the antecubital fossa and the procaine is distributed in its immediate vicinity. There is absolutely no risk attached to an intra-arterial injection into this vessel, since it leads only to the periphery. Also, perineural injection to the radial, ulnar, median, and musculocutaneous nerves will improve the treatment. 7. Femoral Artery*** Indications All kinds of circulatory disturbances affecting the lower extremities, including arterial occlusion disorders (arteriosclerotic and diabetic gangrene) and varicose ulcer; also in nocturnal cramps in the calves, phlebitis, post-thrombotic conditions, angiospastic dysbasia, and impotence. After giving procaine for the above indications, we also like to administer an ozone-oxygen mixture: cellulose pad, hold needle firmly, arrange for assistant to change syringes quickly. This injection is also used in tuberculosis of the bone or joints of the lower extremities (in addition to the usual measures), in order to improve tissue nutrition and thus improving the body’s natural defenses against the tubercle bacillus. Dittmar pointed out that perivasal injection to the femoral artery not only stimulates the blood supply to the regions supplied by this artery and relaxes reflex muscular hypertonicity, but that via the “transition segment” L2 it also develops a therapeutic effect on the lower portion of the large intestine and the whole of the urogenital system. When injecting procaine intra-arterially, followed by insufflation of an ozone-oxygen mix, it becomes noticeable that the increase of blood supply is extended beyond the injection site, in direction of the blood flow. After the infiltration, a vascular spasm occurs and the extremity turns pale and cold. Now the reactive vasodilation takes place. At first, hyperemic red spots appear on the skin, they begin to connect and turn the entire skin red along the vascular area. Cranially the hyperemia extends in direction of the blood flow to the umbilical level! This explains the effectiveness of the injections with → impotence (Leriche syndrome). In the course of treatment of the lower extremities, it can be measured that the area of blood supply disturbance moves distally. This shows that a collateral circulation has formed and regulates the blood supply. Materials About a 40 mm-long needle, not too thin. Quantity 2–3 mL. In an emergency, it is also possible to mix the local anesthetic with Priscol, Ronicol, Actihaemyl, or similar. Technique The patient lies on the examination couch, the legs opened slightly and rotated outward. The femoral artery is now immediately below the inguinal ligament in the fossa ovalis and can be readily found by palpation. The vein lies in a medial direction from it and the femoral nerve lies further laterally. Fix the pulsating artery between the tips of the index and middle fingers. The right hand guides the needle with a short jab almost vertically into the vessel. Aspiration is only very rarely necessary, since blood pulsating into the syringe under its own pressure usually shows when the needle is in the correct position. About 2 mL of procaine are now injected rapidly into the artery. On withdrawing the point of the needle, another l mL is given into the immediate vicinity of the artery. For the femoral artery, the intra- and periarterial injections are combined in order to reach the sympathetic fibers of the adventitia, the media, and the intima, i.e., the whole of the sympathetic reticulum of the artery, which is co-responsible for pathological vasoconstriction and other disturbances. The injection site should be kept compressed for a few minutes after the injection, using a pad, in order to prevent the formation of a hematoma. Fig. 3.5 Injection to and into the femoral artery. The vascular wall is not damaged even if the intra-arterial injection has to be repeated several times. If dark venous blood is aspirated, the needle has been placed too far medially; and if the patient feels an electric flash going down the leg, it is too far laterally and in the femoral nerve (mnemonic IVAN: inside, vein, artery, nerve). Also refer to Fig. 3.17. After the infiltration, the patient perceives a pleasant sensation of warmth in the affected extremity, sometimes in the entire body. Patient and practitioner can recognize the improvement of the dysbasias by the expansion of the distance that the patient can walk without discomfort. (See Fig. 3.5.) The fossa ovalis is subcutaneous and quite near this is the cribriform fascia, a membrane with perforations rather like a sieve. Here there is a large mass of lymphatic and blood vessels, accompanied by a thick reticulum of autonomic fibers. It is palpable as a depression in the subcutaneous tissue. Due to an accumulation of receptors, this is an important site for producing distant reactions. We therefore like to inject a few drops subcutaneously into this area. These should be distributed by short circular massaging movements. 8. Posterior Tibial Artery* Indications Acupuncture has recommended this artery to us for ancillary treatment of disorders affecting the hip and knee joints, the urogenital system, in circulatory disturbances affecting the legs, and particularly for menstrual disturbances. Materials Size 1 needle or longer. Quantity l mL procaine solution. Technique Entry on the inside of the tibia below the calf. Insert the needle as far as the vessel, i.e., until the patient feels a dull pain. Before withdrawal, also give a few drops para-arterially. (See Fig. 3.6.) Fig. 3.6 Injection to the posterior tibial artery. Reversant therapies gain importance in light of the increasing number of patients with regulation disorders or regulation blocks. In addition, the growing interest in biological healing methods has brought the attention of patients and physicians back to autohemotherapy. It can be recommended as a simple and harmless addition to the repertoire of every neural therapist. It can improve the patients own → immune response and vegetative constitution. Some therapy-resistant disorders cause autosensitization through morbific agents or other factors (e. g., metabolic waste, mercury fillings). Often, the irritants cannot be identified and the number of allergens is growing by the day. Specific desensitization can take place only if the irritants that affect the autonomic basic system are recognized and turned into allergen extracts. The patients’ blood definitely contains all sensitizing substances. Autohemotherapy makes use of the specific antibodies formed by the patients’ immune system and the information resulting from previous therapies. Thus, it is more than merely parenteral protein therapy. During hemolysis, antibodies specific to the individual are released. The products of this decomposition stimulate the immune system and increase humoral and cellular defense mechanisms. For example, erythrocatalysis stimulates increased blood formation in the bone marrow, the cytolysis of leukocytes releases immunoglobulins. Stimulation of the storage activity of the reticuloendothelial system (RES) and cell activation has been documented. More phagocytes are produced and the functions of the spleen, lymph nodes, and tonsils are stimulated. It is presumed that the allergen antibodies are processed as a form of countersensitization. All this signifies profound reversal of the autonomic constitution and reactivation of inhibited self-healing abilities. Indications General reversal, furunculosis, carbuncle, pyodermia, acne and other skin disorders, chronic diseases of the respiratory tract, allergies, rheumatism, joint disorders, ulcerative colitis, ventricular ulcer, cerebral sclerosis, and more. Technique Initially, 1–2 mL of blood is taken from the patient’s cubital vein and intramuscularly re-injected immediately. Every 2–3 days the amount is increased by 0.5–1 mL, depending on the patient. In the course of the next 2–3 weeks the amount cannot exceed the → fever threshold of 5–8 mL. The patient has to be informed that he or she might experience a febrile healing response for 1–2 hours, which will disappear by itself. Usually the patient does not require sick leave but should also be made aware that interference fields can be activated and that he or she needs to report every reaction to the physician. In order to increase the therapeutic effect, it does not cause harm to add biological stubstances that stimulate the immune system, such as Elpimed, Echinacin, Engystol, etc. The same applies to ozone-autohemotherapy, where an ozone-oxygen mixture is added and in smaller amounts re-injected; in larger amounts (50– 100 mL) it is reinfused after adding citrate. There are additional therapies, including the modified autohemotherapy according to Theurer, hematogenous oxidation therapy, and the use of blood that has been potentiated or treated with ultraviolet radiation or hemolysis. This radical reversant method should probably be used only as in-patient hospital treatment, since extensive irritation can result. Materials Lumbar-puncture needle with mandrin or 0.8 mm diameter 80 mm-long needle, 10 mL record syringe. Amounts We slowly inject 1 mL of 1% procaine solution from an ampoule without additives intrathecally into the liquor at the end of the pump operation. Before this, to avoid excessive irritation to the liquor-forming system, we first draw off about 8–10 mL of liquor. In the original method, only the liquor itself was pumped back and forth without the addition of procaine. Contraindications Hypertension, tumors in the posterior cranial fossa, central vascular disorders. Technique Absolute sterility is essential: disinfection of the skin, sterile surgical gloves. Lumbar puncture The patient sits astride a stool. His or her head is held firmly by an assistant, bent slightly forward. Preferably, the patient should put his or her arms around the assistant. The needle is inserted between the spinous processes of the third and fourth lumbar vertebrae at the point of intersection of the line connecting the iliac crests with that of the spinous processes. A → (T) quaddle is set over the point of entry and the needle is then guided first straight ahead and then its point should continue slightly upwards and in a perfectly sagittal direction until it meets an elastic resistance. When it has passed through this, the cannula is in the correct position and liquor will drip from the needle. The needle is left in position and the syringe attached to it. Liquor is now aspirated into the syringe and the 10 mL of liquor is re-injected. The process is repeated 10 to 20 times, so that a total of 100– 200 mL of liquor is moved. Side-effects Headache, vomiting, nausea. These symptoms can to some extent be controlled with analgesics. The removal of a quantity of liquor at the end of the treatment can considerably reduce these side-effect symptoms. Years ago, I was able to cure a woman of flaccid paralysis of both legs, which had persisted for 2 years, by means of three such liquor-pump treatments with the addition of 1 mL of Impletol, at intervals of 3 weeks. On each occasion I removed 10 mL of liquor as a prophylactic measure to relieve pressure. Caution: Indications This method is not suitable for general practice! It should be used only by the experienced physician and if the possibility of in-patient treatment is available. It must never be used unless segmental therapy and the search for a possible interference field have proved fruitless and even then only if there is a sufficient number of subjective and objective symptoms suggesting a possible interference field in the cerebral region. Obviously, the principle that the seriousness of the clinical picture must always be in a proper relationship to the risk attached to the therapeutic intervention also applies to this injection. In the case of a cisternal injection, the risk resides less in the injection of procaine as such than in the suboccipital puncture it necessitates. The percentage of cures due exclusively to treatment based on this diagnostic procedure is certainly not very high. Nevertheless, the risk attached to a diagnostic suboccipital puncture seems a reasonable one to take. Before Huneke, pharmacologists prophesied that fatalities would result from the intravenous administration of procaine. Today, no one thinks of this and the extensive literature on the intravenous procaine injection unanimously praises its many-sided therapeutic effect. I am therefore prepared to prophesy that the same will be the case with regard to the cisternal injection of procaine, and that it will gain a firm place in therapy once the theoretical doubts and its own inevitable teething troubles have been overcome (Case Histories 16–18, Part I, Section C). In the case of severe systemic disease, organic cerebral damage with loss of tissue, cerebral atrophy, multiple sclerosis, amyotrophic lateral sclerosis, and pyramidal signs (positive Babinski reflex), the therapy is without prospect. Objective symptoms: • Positive Romberg test. • Impaired ocular convergence. • Dysmetria (signs of ataxia, finger-nose test). • Snout reflex according to Wartenberg (“the Babinski of the head”): the reflex is positive, if when tapped with a reflex hammer the loosely held lips are arched forward like a snout. • Eye test according to Reid: the patient faces straight ahead and his or her eyes follow the physician’s finger moving to the extremities of vision to the left, right, up, and down. The reflex is positive, if a fine tremor of the head results, which may be accompanied by dizziness or nausea. • Wartenberg’s head-retraction reflex: the patient bends his or her head slightly forward. The reflex is positive if the head is briefly retracted when the upper lip is tapped with the reflex hammer. • Other pathological reflexes, e.g., absence of the abdominal-wall reflexes. If several such signs are present simultaneously, the cisternal injection is justified. Anatomy The cistern is the cavity between the pia mater and the arachnoid. It is filled with liquor and opens into the fourth ventricle and, indirectly, the entire ventricular system. Almost all the cerebral nerve centers lie in the rhomboid fossa, on the floor of the fourth ventricle, including the autonomic centers of the vagus, the glossopharyngeal and the intermedius nerves. They are particularly important for respiration and circulation. By way of the cerebral aqueduct, the fourth ventricle communicates with the third ventricle of the diencephalon. There is also an indirect connection from there to the pituitary gland. The “pituitary-diencephalon-system” comprises the primary center for autonomic regulations. Materials The needle should preferably be a suboccipital-puncture needle with a mandrin, or a normal l mm diameter 80 mm-long needle; in addition, an empty 10 mL syringe and a 5 mL syringe containing l mL procaine will also be required. Technique If possible, the patient should have an empty stomach. He or she is seated backwards astride a chair, and supports their head on their folded arms resting on the back of the chair. An assistant firmly holds the patient’s head bent forward to prevent it from jerking back. The hair is cut away over the suboccipital site, between the spinous process of the second cervical vertebra, which can be readily found by palpation, and the squamous part of the occipital bone. The area around the site is cleaned with soap and water and thoroughly disinfected. The needle is inserted (see Fig. 3.7) above the spinous process of the second cervical vertebra, one to two fingers’ breadths below the squamous part of the occipital bone, at first exactly in the sagittal plane. It is then directed obliquely upwards through the nuchal ligament until its point reaches the lower edge of the squamous part of the occipital bone and should just be able to slide through under this. At a depth of about 70 mm, the elastic resistance of the atlanto-occipital ligament can be felt. After passing through this membrane, the needle must not penetrate more than 5 mm into the cistern, lest the medulla be punctured! A lesion to the center for respiration will most likely cause sudden death. To make certain that the sudden jerk following penetration of the membrane can be halted at once, the physician places his or her right hand flat on the back of the patient’s head and his or her left on the haft of the needle in such a way that it acts as a brake. Fig. 3.7 Injection into the cistern. Before the injection, the patient should be warned of a number of reactions, some of which may be unpleasant, and the physician should remain with him or her for 20–30 minutes until they have subsided. Soon after the injection, the patient’s pulse rate will rise and he or she will feel nausea. This will be followed by a sensation of warmth and an outbreak of sweating. There may also be coughing, sneezing, or violent yawning, and hallucinations of taste or smell may occur. Following this reaction of the sympathetic nervous system there will be a parasympathetic phase with a slowing of the pulse, pallor, a feeling of cold and nausea. In case of severe decrease of blood pressure, Trendelenburg’s positioning (head slightly lowered, pelvis and legs elevated), which normalizes blood pressure quicker and more reliably than short-acting analeptics, should be used. Due to the concentration and amounts used in neural therapy, a paralysis of the respiratory center is improbable. According to the Americans Koster and Kasman, the respiration center possesses characteristics that make it insensitive to the amounts of local anesthetics used in spinal anesthesia. Depending on the autonomic state at the outset, the type, severity, and duration of these autonomic reactions may vary. They disappear after anything from a few minutes to 30 minutes, when the patient’s equilibrium is restored to normal. Sometimes this injection can be given with hardly any reactive symptoms by the patient, at other times the same patient may present with severe, even excessive reactions. But no case of damage or fatal sequelae has been reported. Following the injection, the patient should remain recumbent and under the physician’s observation for at least an hour and should not be discharged except under observation. If possible, he or she should rest in bed for a couple of days. For these reasons, in-patient treatment is desirable. If, during this period, there are symptoms of meningeal irritation such as a rise of temperature, headache, and a hint of a stiff neck, the prescription of analgesic suppositories is all that will be required. Theoretical doubts have been voiced against the cisternal injection of procaine, some of them very weighty indeed (Janzen, Lendle, Moellhoff, Zipf) and, as a result, the Federal German Medical Association has issued a warning against using this injection. Lendle issued a massive threat by stating that “in case of accidents, a person who has used this form of therapy cannot expect to find any grounds based on theoretical and pharmacological considerations to relieve him of responsibility.” He regards it as theoretically impossible that l mL of procaine injected into the cistern will not produce paralysis of the respiratory center, collapse, etc. Against this, however, there is the fact that in practice, involving several hundred such injections, there has never been a serious incident, much less a fatality; there have, on the other hand, been cures that could not be previously obtained by any other means. If theoretical objections had always carried the day in the past, there would never have been such things as railways, airplanes, or nuclear reactors, not to mention a large number of surgical operations. The risk of the cisternal injection lies entirely in the suboccipital puncture, in which one cannot, of course, ever be absolutely certain that there will be no complications as a result of cerebellar tumors and vascular abnormalities. Nevertheless, in neurology it is carried out for purely diagnostic purposes as an almost daily routine and with little hesitation. The introduction of procaine into the cistern does not in our experience constitute any significant additional risk. Nonetheless, it is advisable always to have a cortisone preparation in readiness for intravenous administration in case of shock or collapse, such as Solu-Decortin H or Ultracorten H (water-soluble). Alternative terminology Extradural, peridural, or sacral anesthesia, caudal block. Anatomy The injection into the sacral canal from the sacral hiatus reaches the epidural region (epidural space, also called extradural or peridural space), which extends superiorly to the foramen magnum, which the dura is attached top. A connection to the liquor-containing space does not exist! The epidural region is located between vertebral canal and dura, and surrounds the dural sheath down to the second sacral vertebra, where the dural sheath ends. From this point caudally, it only contains (in addition to the external filum terminale, which attaches the dural sheath caudally) the coccygeal segmental nerve and the sacral segmental nerves (S1 through S5). Their anterior branches travel through the pelvic sacral foramen and their posterior branches through the dorsal sacral foramen. Caudal (epidural) anesthesia blocks the areas that are supplied by these segments. Different from → (T) presacral infiltration, this also blocks the posterior roots. It enables us to achieve an anesthetic (i.e., neural-therapeutic) effect in the following areas: • skin: anus, perineum, scrotum, penis, and the region of “saddle-block anesthesia” of the lower buttocks; • organs: lower rectum, vagina as far as the cervix of the uterus, ureter, pelvic floor, prostate; in addition, the anal sphincter is also relaxed. The spinal nerves that pass through the dural cavity are surrounded by thick dural sheaths. Thus, in order to produce a “saddle-block anesthesia” adequate for surgery, we need to use substantial quantities of anesthetic solution, as in any epineural conduction anesthesia (about 20 mL). The epidural space lies between the dural sheath of the spinal cord and the periosteum of the vertebrae and can hold over 100 mL. For neural-therapeutic purposes it is often sufficient to inject 5 mL of procaine, and this is soaked up by the loose tissue in the epidural cavity as if by a sponge. Experiments have shown that this solution can diffuse as far as the cervical segment. Indications Any disorders in the areas mentioned above, irrespective of whether they present as inflammation, pain, itching, or other symptoms, principally affecting the external and, in part, also the internal genitals; sexual disturbances, enuresis, bladder disorders, encopresis in multiple sclerosis, piles. Obstetrics form a special indication. Epidural anesthesia with 20 mL of procaine will, after about 15 minutes following administration, reduce labor pains for 1–2 hours without in any way inhibiting labor. On the contrary, expulsion of the fetus is facilitated and accelerated by the relaxation of the pelvic floor. Reischauer explained the effectiveness of epidural and presacral infiltrations in the treatment of sciatica by their ability to damp effectively the reactions of the spinal roots and the sympathetic fibers in their vicinity, which are due to the mechanical irritation caused by the prolapsed cartilaginous disks. Materials 1 mm 60 mm-long needle. Quantity Depending on the object of the injection and varying from 5–20 mL of procaine solution; 10 mL remain mainly within the epidural cavity of the sacral region, 20 mL diffuse as far as the lowest lumbar segments, 30 mL as far as the level of the umbilicus (T9 to T10) and 40 mL will spread as far as T6 to T7 (lower shoulder blade). Even if as much as 40 mL of 1% procaine solution is injected, there will be no motor dysfunction of the legs, which would complicate outpatient treatment. Technique The knee–elbow position is generally recommended but is not essential. A number of authors prefer the patient lying on his or her side. We prefer the patient to stand hard against a table, placing the upper part of the body bent at right angles forward on the table top. The finger is used to palpate the readily identifiable bony protuberance of the sacral cornua and the springy membrane stretched between them that covers the sacral hiatus. This lies about 20 mm above the cranial end of the natal cleft. It is occasionally difficult to locate this, but only in adipose patients. In such a case, it is merely necessary to go about 40–50 mm cranially from the tip of the coccyx to find the opening. After disinfection of the skin, the 60 mm-long needle is inserted steeply into the upper part of the membrane; the haft is then depressed far enough to enable the needle to slide a further 40–60 mm cranially within the sacral canal. Fig. 3.8 Epidural (sacral) anesthesia. Fig. 3.9 Diagram showing the epidural injection. 1. Entry of the needle through the upper part of the membrane. 2. The needle is depressed a little and advanced about 50 mm into the sacral canal; the injection is given following a negative aspiration test. Aspirate (there must be no sign either of blood or liquor)! The dural sheath ends approximately 60– 90 mm above the point of insertion of the needle and thus it will not normally be penetrated. If the needle is in the correct position and the injection is given slowly, the procaine should flow out without resistance. One can also check that the needle is in the correct position by injecting about l–2 mL of air after negative aspiration. If an emphysema forms in the skin, the needle is not in the sacral canal. If it is in the right position, the air injected into the sacral canal will push the subarachnoid away from the point of the needle. If (on rare occasions) the dural sheath is, in fact, entered, refrain from giving the injection that day. There are no other unpleasant side-effects from this injection and it offers hardly any technical difficulty. Because of its wide spectrum of indications, it is thoroughly recommended for general practice. The effect can be further increased by additionally injecting into the → (T) sacral foramina. Figures 3.8 and 3.9 show the epidural anesthesia and injection technique. Indications All → abdominal disorders. This injection to the peritoneum of the upper abdomen will often suffice on its own; if it does not, we combine it with one to the abdominal→ (T) celiac ganglion. Materials About a size 1 needle. Quantity 2 mL. Technique The patient lies on his or her back. The entry site (Fig. 3.10) is on the midline of the body, three fingers’ breadths below the xyphoid process, and we penetrate to a depth of 30–50 mm, depending on the adiposity of the patient, to the linea alba, infiltrating a little all the way. There is noticeable resistance as we penetrate the fascia. Immediately beyond this the point of the needle is at the required injection site. Even if we were to penetrate the peritoneum accidentally, the slow progress of the needle under constant pressure on the plunger and with steady infiltration as we proceed would prevent injury to the abdominal organs. In 1976, a court in Esslingen convicted a non-medically trained practitioner on a count of negligent manslaughter of a woman patient who died of necrosis of the pancreas following an injection into the epigastrium. During surgery it was found that not only had the lobe of the liver been punctured, there was injury also to the pancreas! This singular case indicates too brusque an approach with too long a needle and a lack of anatomical knowledge. Fig. 3.10 Injection into the epigastrium (to the upper part of the peritoneum). Alternative terminology Paracervical block, transvaginal injection to the utero-vaginal plexus (Frankenh user’s), or utero-sacral block. Anatomy The plexus of the female pelvis travels along the interior iliac artery and the uterine artery to the lateral uterus. In its parametrium it forms the large utero-vaginal plexus (Frankenh user’s) with many ganglia. Secondary fibers of the pelvic plexus innervate the bladder, vagina, the uterus, and the clitoris. Indications 1. Segmental therapy: For endometritis, parametritis, dysmenorrhea, abnormal menstruation, neuritis of the pelvic floor, lower abdominal pain and/or backache, dyspareunia, sense of pelvic discomfort and downward pressure, frigidity, sterility, disorders connected with the menstrual period such as headaches, autonomic pelvic disorders etc. In 1954, Goecke reported more than 247 cases of cervical hypersecretion that did not respond to any form of treatment. In 83.3% of the cases he was able to cure or considerably improve the condition with paracervical infiltrations. This also cures existing portio erosions. In the treatment of → irritable bladder, urination regulating parasympathetic and sympathetic fibers of the pelvic plexus are temporarily blocked. For this condition we also infiltrate paraurethrally at the anterior vaginal opening, 2 cm dorsally to the external vesical orifice. b. In searching for an interference field, as a test injection: When the patient’s history includes: vaginal discharge, abortions, difficult labor, pregnancy terminations, D&C, puerperal fever, pelvic inflammatory disease, gonorrhea, any surgery involving the genitals. The test injection into the → (T) pelvic cavity through the abdominal walls is much easier and generally adequate. If this produces a substantial improvement in the remote disturbances but does not achieve the required 100% freedom from symptoms, the transvaginal injection to Frankenhaeuser’s plexus should be tried in addition. c. In labor: In obstetrics, 5–10 mL of procaine solution are given on each side to relieve pain in the initial phase of normal, straightforward labor. In first pregnancies it is advisable to wait until the cervix has reached a diameter of about 50 mm; in subsequent pregnancies it should have become dilated to about 30–40 mm before the anesthetic is given. The first side is infiltrated between two contractions, and the other infiltration given two contractions (i.e., about 15 minutes) later. The pain-relieving action generally starts to take effect immediately and remains effective for 1– 2 hours. Infiltration of greater quantities blocks the pelvic plexus with its links to the presacral nerve and to the sacral plexus. Contraindications During the period, it is advisable to avoid any transvaginal intervention, including this injection. However, there is no objection even during menstruation to an injection from the outside into the → (T) pelvic cavity. Materials 0.8 mm 80–100 mm needle, with pilot tube like the PP needle made by Woelm; quantity 2– 4 mL, 10–20 mL for obstetric purposes. Technique The bladder should be emptied before the injection. The patient lies on the gynecological couch and the uterine cervix is fixed in a speculum. On psychological grounds one should avoid, as far as possible, letting the patient see the long needle. Insert the needle through the mucosal fold in the lateral fornix beside the cervix, i.e., through the lateral vaginal vault, between about the 3- and 4-o’clock positions and the 8- and 9-o’clock positions. The needle is then advanced slightly at an angle in a lateral and dorsal direction. The cervical ganglia lie laterally from the cervix and further dorsally. We now distribute about 1–2 mL procaine on each side, at a depth of only about 10–20 mm, to Douglas’s peritoneum and to Frankenhaeuser’s plexus (see Fig. 3.11). The risk of puncturing the ureter or the uterine artery is easily avoided by going in as described through the vaginal vault rather than advancing parallel to the cervix. This injection influences the parasympathetic pelvic ganglion, which lies near the organ and has important regulating functions. Fig. 3.11 Injection to Frankenh user’s ganglia. The point of entry of the needle is through the lateral fornix, injecting at a depth of 10–20 mm. When working with the needle with a pilot tube, the free hand locates the point of insertion of the needle between index and middle finger. The free hand then slides the tube to this point and places its outer end on the inside of the opposite thigh. The assistant introduces the long needle with the 5 mL syringe attached into the pilot tube without letting the patient see it. The needle is inserted as far as it will go, aspiration is carried out under the doctor’s control and 2 mL of procaine injected. The same injection is then also given on the other side. Consequential bleeding is very rare. If it occurs, it can be stopped by compression with a cottonwool pad covered with lint. The patient remains lying down for 15 minutes. After a check, an absorbent pad is placed in position, and the patient is allowed to leave. Gynecologists are used to hooking into the posterior external os of the uterus with bullet forceps to pull the portio toward the symphysis in order to inject the local anesthetic into the tightened sacrouterine ligaments. Our experience has shown that this injection can be successfully done without hooking into the portio. However, if doing so, one should also remember to inject into the attachment site of the forceps in order to avoid scar interference field formation. Any scars present as a result of a perineal tear or episiotomy are of course always injected at the same time. In women who have already had children, the perineum should always be infiltrated at least once at the same time as giving this injection, even if there are no externally visible macroscopic scars. The injection into the perineal tissue is painful because of the large number of nerves it contains. Scars following Emmet’s tears and in the related parametric ganglia so often form interference fields that they must be found by palpation and treated by injection. Here, too, the PP needle is used to help locate the point of insertion accurately. If the scar tissue is so hard that the needle is difficult to insert, it helps to ask the patient to cough, and the needle will enter effortlessly. It is worth taking a little trouble over this injection, since it will often be rewarded by a genuine Huneke phenomenon. For any vaginal interventions that may become necessary in virgins, the intact hymen can be made soft and flexible enough for the speculum for about 24 hours by mixing an ampoule of kinetin (hyaluronidase) with 5 mL of procaine solution, first infiltrating under the base of the hymen with a fine needle and then infiltrating directly. Anatomy The intercostal nerves supply the sensory nerves to the thoracic wall including the parietal pleura and the anterior abdominal wall with the sensitive parietal peritoneum. From the intervertebral foramen to the angle of the ribs the nerve runs in the middle of the intercostal space, from there to the anterior axillary line it lies directly under the rib. If the effect of the injection is inadequate, anastomoses of the intercostal nerves make it necessary to block the adjacent nerves at the same time. Indications Intercostal neuralgia, shingles, carcinoma pain. To relieve pain and improve respiratory excursions and the coughing up of secretions in fracture of the ribs, pleurisy, pulmonary embolism, pneumonia and following abdominal surgery; pectoralis minor syndrome. If the patient is suffering persistent pain, the possibility of vertebral disorders, cancer, and tabes should also be considered. Materials Short needle. Quantity Procaine solution per injection: 0.3–1 mL. Technique By inserting the needle 50 mm to the side of the spinous process and going straight down, one strikes the relevant rib. Obviously, we look for this nerve according to the site of pain, e.g., next to the spine, in the axillary line or next to the sternum. If we want to inject next to the spine, the patient needs to place their forearm onto their forehead, which moves the shoulder blade away from the place of insertion. In this section, the nerve is still located in the middle of the intercostal space. In order to affect the lateral cutaneous ramus, insertion into the posterior axillary line is recommended. Then we advance the point as far as the lower edge of the rib. To avoid injury to the pleura we maintain steady pressure on the plunger and continue another 5 mm—never more!—into the intercostal musculature. In doing so we try to obtain nerve contact. The patient needs to be prepared for the sharp pain this will produce, to avoid making any sudden defensive movement due to fear or surprise. If their symptoms recur, they will probably happily submit to this somewhat painful injection again, for nothing else can better relieve or get rid of these unpleasant and frightening symptoms. If very occasionally the pleura is accidentally perforated, a pneumothorax may be produced. Generally, this is not even noticed by the patient, but if it is, it will heal of its own accord within 2–3 days with a little care on their part to avoid overexertion, but without substantially affecting their general state of health. If more severe symptoms continue or if there is any suspicion that they might have a tension pneumothorax, an x-ray check is advisable. Intra-arterial injection → (T) afferent arteries. Indications Conditions following x-ray or radium therapy; in addition to the injection into the → (T) pelvic region and to → (T) Frankenhaeuser’s ganglia for any sequelae of febrile abortion, placenta accreta, cesarian section, endometritis, cervical stump following a supravaginal hysterectomy, cervical tears after labor, conization, cervical erosion etc. Materials 1 mm diameter 100 mm-long needle, a self-retaining speculum, vulsellum, tightly fitting record syringe. Quantity 4–6 mL. Technique The injection described by Mink requires good fingertip sensitivity, patience, and the ability to proceed with gentle force. In addition, because it can be painful, it also calls for the ability to distract the patient verbally. The patient is placed on the gynecological couch and the speculum adjusted to visualize the cervix. Following disinfection, the anterior lip of the cervix is carefully grasped with a vulsellum and pulled forward. The 100 mm-long needle must not be too thin. This is now inserted as far as it will go in the cervical canal. When resistance is felt, the point of the needle should be advanced into the myometrium by slow screwing motions through the mucosa of the isthmus or the body of the uterus. One can also use a simple trick to get the point of the needle into the uterine musculature without the patient being aware of it: hold the syringe firmly, ask the patient to cough briefly, and the needle will be in position. Aspirate, then patiently, sensitively, press middling hard on the plunger of the syringe. It may take as long as 2 minutes before the reactive spasm of the uterine musculature relaxes and the preparation can flow in relatively easily. In sensitive or highly-strung women it may first be necessary to give a paracervical injection to → (T) Frankenh user’s ganglia. There is no need for a general anesthetic. Following an intramural injection into the uterus, we also inject the site where the vulsellum was attached in the cervix, and after overcoming the resistance of the musculature we allow another l–2 mL procaine to flow into the myometrium from there. Following the injection, the patient inserts a vaginal tampon to absorb any bleeding that may occur on her way home. This injection is repeated at intervals of several days to a week. As a rule, three to five injections will suffice, even for the main indication of reactive fibrosis of the endo- and myometrium following radiation treatment for carcinoma. We regard an intramuscular injection merely into the upper outer quadrant of the buttock as a complete waste of time, even when, as in Aslan’s therapy, it involves a continuous flooding of the organism with large amounts of procaine. Ours is a selective procaine therapy and we therefore limit ourselves to looking for pathologically changed muscle tissue for treatment by our method. Every disturbance of the organism affects the skin through viscerocutaneous pathways and the muscles through visceromuscular pathways (see Fig. 1.2, Part I, p. 24). More thorough (deeper) palpation of the painful muscles guides us to particularly painful points, the so-called myofascial → trigger points. They emit centripetal pain impulses. If the centrally regulated reflex responses fail because of positive feedback, the pain keeps intensifying retroactively and more and more peripheral nociceptors are engaged. The anesthesia of the hyperalgesic points and zones interrupts the vicious circle. It eliminates all local disturbances and those originating here but traveling to other areas. Complicated processes of movement, such as physical work, sport, ball games, walking, dancing, etc., are based on the collaboration of connected muscle groups, so-called myokinetic chains. Pain in some of the muscles can be transmitted to others and spreads. When we eliminate the source of the pain in muscles, tendons, and periosteal attachments, we also eliminate the pain that has traveled from there to other places. It has been proved that the injection of procaine into spastic musculature whose nutrition has been disturbed reduces its tonicity and pH. By eliminating pain we can also interrupt pathological reflex processes. This explains the disappearance of locally circumscribed and of more extensive disharmony. The tissues treated with procaine are better supplied with blood. This stimulates the metabolic exchange and thus the removal of metabolic waste, which is a pain-producing agent. If the reflex muscle spasms improve together with the attenuation of pain, the patient becomes psychologically more relaxed. This activates the highly desirable cooperation on their part that results in further improvement in their muscular functions, enables them to cope more easily with the dysfunction, and become less dependent on medical aid. Hence, our motto must be to make the patient pain-free and encourage movement. If muscular stiffness persists for too long, there is a risk that it may turn into an irreversible condition with the formation of mature scar tissue and completed cicatrization, and create an interference field. Indications Trigger points, myalgia, lumbago, muscle spasm, muscle strain, muscle tears, myogelosis, fibrositic nodules, torticollis, vertebral syndrome, tension headache, bad posture, unilateral physical stress through work or sport, insertion tendopathy, muscle tension due to stress; also all segmental muscle-reflex symptoms, including joint and organ disorders. Materials We choose a needle according to the depth at which the process is taking place. Since this is often greater than at first assumed, it is always better to use a longer needle. Quantity The quantity of procaine used will depend upon the extent of tissue change, but we never inject at the rate of more than a few drops to a tenth of a milliliter of the product in any one site. Technique We set a → (T) quaddle over the site indicated by the patient or found by palpation to be painful and then pass the needle through this to probe deeper. When the diseased muscle tissue is reached, the patient will indicate this by reporting a sharp pain (“ouch point”). Anyone who has developed a feel for tissue will sense that the needle is meeting resistance in the altered tissue. There is a kind of creaking sensation as if one were going into sandy clay. Into this area we inject only a few drops of procaine; these will be enough to set off the required healing reaction. We always need to limit ourselves to working with the smallest possible stimuli. The improvement does not depend on the anesthetic effect. What matters is not so much the quantity as the correct site! After the injection, we distribute the product through the tissue with a few circular massaging movements. The injections may need to be repeated after a few days. There is no better or economic form of treatment than this. Indications Intravenous injections of procaine are used: • to relieve pain (as endoanesthetic); • as vasodilator; • to regulate the circulation; • as ganglioplegic; • to reduce vascular permeability; • as anti-emetic; • as diuretic; • as anti-pyretic; • as spasmolytic; • as anti-allergic measure (as anti-hyperergic); • as autonomic reversant treatment; • to reduce inflammation (as anti-phlogistic). By way of the blood vessels, the intravasally injected or enterally administered local anesthetics reach the internal and external sensory organ and tissue receptors or afferent structures and desensitize them by decreasing their excitability. The synapses of the parasympathetic ganglia respond to lower doses of the anesthetics than the sympathetic ganglia. This “endo-anesthesia” affects, for example, the receptors of the carotid sinus and the heart and inhibits stimulus formation and transmission in the cardiac muscle. We also reach the visceral receptors of stomach, intestinal, and urogenital system, as well as muscle and pain receptors this way. The favorable effect of procaine on cell metabolism is based on an increased oxygen supply and the more rapid removal of metabolic products. It sensitizes the uterus to the posterior pituitary hormone. In the experimentally induced circulatory collapse using veratrin (Bezold-Jarisch reflex; Eichholtz, Fleckenstein, Muschaweck, Zipf), a spontaneous lowering of blood pressure occurs, with a reduced pulse and respiration rate to the point of collapse. We know this reflex clinically in the shape of a cardiac infarct. The collapse response can be prevented by giving procaine intravenously, because this desensitizes the receptors. The same also applies to anaphylactic serum shock and necrosis formation in the Shwartzman-Sanarelli phenomenon (Hirsch, Keil, Rademacher, Siegen). Through experiments, these authors also showed that the permeability of terminal blood vessels due to dysregulation can be reduced. This imposing list of positive characteristics has earned procaine its title of “king of medicines.” But in addition to this, procaine or lidocaine given intravenously develop an effect so extensive that none of the attempts made to explain this from a pharmacological point of view can offer full satisfaction. In addition to eliminating vasomotor dysregulation, there can be little doubt that it also has a regulating effect on higher order control centers. It improves the patient’s general state and restores the balance in all autonomic dysfunctions. In doing so, it will depend on the initial state whether it acts as an autonomic relaxant or improves tonicity in the opposite sense. This neural-therapeutic effect tending to re-establish the normal state is set off by the healing stimulus that procaine has upon the autonomic plexus of the vascular wall. This effect is obtained with even the minutest quantities of procaine. We therefore take the view that if l mL is not enough to produce a decisive response from the reticular system, then 5 mL or even more will not do so either. But since with intravenous injections the risk of toxicity increases rapidly with the quantity administered, any larger quantities are as dangerous as they are pointless. Thus, let us stick to the single and harmless milliliter. The intravenous injection of l mL of procaine and of a few drops given paravenously, in conjunction with injections to other sites, is indicated as a reversant and balance-restoring basic treatment for all disorders in the regions of the head, neck, and throat and of the thoracic area, and for the treatment of a large number of other pathological conditions. It affects regulation centers and vasomotion. Repeated injections have a regulating effect on the autonomic system and act as a normalizing vascular exercise for all neurovascular dysregulations and their consequences for trophism and function of the tissues: The effect of intravenous anesthesia on the functions of the autonomic nervous system can be compared to the sympathetic blocks and the vegetative drugs used with these disorders. Sympathetic blocks and intravenous anesthesia have a longer lasting effect than the duration of the actual anesthesia (reversal of the autonomic tonus). (Leicher, Haas) In South America, surgical operations have been carried out since 1950 on patients placed under an anesthetic induced by the intravenous administration of procaine. A barbiturate is given as a basic narcotic, together with a muscle relaxant, and 1% procaine is then administered in a 5% dextrose solution by continuous infusion. This anesthetic has been found to be adequate for most surgical operations! A report by Parada covered 300 000 cases and showed that this method is well-established and widely used. Materials Size 12 needle. Quantity Only 1 mL of 1–2% procaine or 0.5–1% lidocaine solution. We may inject a larger amount only under exceptional circumstances, but always without adrenalin, acetylcholine, or other autonomic stimulants! Never inject more than l mL 2% procaine intravenously! As a matter of principle, any departure from this well-established rule should be treated as a rare exception and must be left exclusively to experienced neural therapists! And not even they should exceed 2 mL and must always inject slowly. Zipf lists as intravenous maximum dose 2.5 mg/kg body weight. A little more could be too much! In a court judgment given in Frankfurt in 1967, it was stated that an intravenous injection in conjunction with other procaine injections is not permissible “in view of the cumulative effect of stimuli.” Our practical experience does not accord with this. Intravenous procaine therapy has a general reversant effect on the autonomic system, which cannot be achieved to a similar extent by subcutaneous or intramuscular injections. Segmental therapy with procaine is used for other purposes. Provided the limiting dosages are observed, the two types are perfectly compatible with each other. Technique Except in very apprehensive or labile patients who are better treated in a recumbent position, this injection can be given without hesitation to the seated patient. Contrary to what one may often read, practitioners of neural therapy according to Huneke do not inject particularly slowly. F. Huneke injected the milliliter into the antecubital vein, with tourniquet in position and then released the tourniquet. Thus, the whole of the dose enters the circulation at a stroke and thus literally constitutes a therapeutic thrust into the neurovegetative system. Before taking out the needle, always also give a few drops of procaine paravenously, as the neural-therapeutic effect is further increased if the reticular system surrounding the vein is included, since this is particularly richly supplied with autonomic terminal fibers. Procaine is rapidly metabolized in the body. In an emergency, the intravenous injection may be repeated at half-hourly intervals. Side-effects Sometimes, following the injection, the patient may show such symptoms as slight dizziness, pallor, tremor, or sudden sweating (“initial vaso-spasm”). All these are perfectly harmless and pass off after a few minutes. When using procaine, we may very occasionally see more severe symptoms such as a tendency to collapse or unconsciousness. In such a case, we lay the patient flat and let them remain like that for 15 minutes. Do not give any vasopressors! In over 100 000 intravenous procaine injections to date, I have never yet had a case of convulsions, coma, respiratory paralysis, let alone a fatality. Reports of such incidents are based on overdoses and on the fact that other and more toxic preparations containing procaine or modern amide-structured local anesthetics with a vasoconstrictor additive have been used! If the patient feels thirsty, he or she should be given a drink of water. Nervous, highly-strung patients should be given a harmless valerian preparation. From a morphological anatomical viewpoint, the joints are merely movable links between the bones. Seen cybernetically, they also have an important control function. Their environmental sensors (receptors) not only indicate to the center the positions of the joints, they also regulate and influence their own environment via the autonomic nervous system and trigger off pain when there is an appropriate stimulus. The interlinking with other control circuits passes beyond the spinal-reflex functional circuit to affect every part of the organism. Our knowledge of these relationships should prevent us from regarding or treating any system—in this particular case the joints—in isolation. With regard to treatment, as far as possible we prefer to do without corticosteroids and medical preparations, since they are mostly as unnecessary as they are harmful. However, we must exclude dislocations, major fractures, articular tuberculosis, and metastases from treatment with our injections or, if neural therapy is used, it should be limited to a supporting role. The standard diagnostic procedure must not on any account be omitted before treatment. X-rays and laboratory reports have in our view a purely informative function, since they can tell us little about the state of the diseased structures and nothing about the causal relationships between the pathological findings and the loss of function. This is particularly true of degenerative changes such as osteophytes and the like. At all events, unusual or even abnormal findings should not deter us from taking appropriate neural-therapeutic action. Injections directly into the joints will only rarely be necessary! Generally, all that will be required are → (T) quaddles and deeper → (T) intramuscular infiltrations near the joint. So, for example, arthritis or arthrosis of the knee due to purely segmental causes will be cured by five simple quaddles around the knee, provided that this segmental treatment is repeated a sufficient number of times. If this treatment does not produce the desired result, then other injections in the segment hold little prospect of success. Nevertheless, it still happens time and again that injections of procaine or Xyloneural into the joint work wonders. Procaine has a desensitizing effect on the synovial membrane. But if there is an interference field at the root of the pathological changes in the joint, neither quaddles nor intra-articular injections will help to any significant degree. We have been taught that there is a certain risk of infection whenever an injection is made directly into a joint. On the other hand, we have learned from experience that procaine, apparently by normalizing the electrical charge, can prevent → infection and → inflammation. This pragmatic discovery is further strengthened by the fact that we have never seen a single case of infection after injecting procaine. With this knowledge we can overcome the fear of infection following the injection of procaine into a joint. But to make doubly sure, we should always disinfect the skin over the joint before giving the injection (for liability reasons too!). This should be done particularly thoroughly if the patient has previously been on (regulation-blocking) corticosteroids, since in such cases the risk of infection is especially high. As always, the needle should be inserted briskly. If the point of the needle can be moved freely, if it is easy to inject air, and if synovial fluid is aspirated, we have the assurance we need that the needle is correctly positioned within the joint capsule. If there is an existing effusion, this should be aspirated and a little of it mixed with the procaine before the injection. Injections of procaine or Xyloneural into joints can be made irrespective of the blood sedimentation rate, the patient’s age, or the length of time that they have suffered from their disorder, and regardless of their general state of health. Even the worst possible x-ray picture is no contraindication. The function is always of far greater interest to us than this momentary photographic record of the skeleton (provided that malignant processes can be excluded). We do not intend to change anything in the bone but to improve, and, as far as possible, restore the function. Our form of treatment is far more sparing than any surgical operation. For this reason, a sufficient number of repeat injections should always be planned before any intended surgery. Before any surgery is undertaken, every possibility in the segment should be exhausted and a painstaking search for a potential interference field concluded. If a disorder is due to an interference field, any local surgery is pointless and is bound to fail. In treating painful joint disorders we need to remind ourselves that the neural and vascular supply of all the major joints (shoulder, knee, hip, elbow) emanates from the flexor aspect of the joint. A deep injection from there as far as the periosteum is always without risk, even if we pass the needle through nerves, vessels, and joint capsules, and even intra-arterial injections are harmless and are often extremely desirable. Since, in giving such injections, we are carrying out segmental therapy, a substantial improvement is all that is required to justify repetition. Indications: Arthritis, arthrosis, gout, all post-traumatic symptoms and partial stiffening of the joint, recurrent effusions. The joints listed below will be covered over the following pages: 1. temporomandibular joint, p. 306; 2. shoulder joint, p. 306; 3. acromioclavicular joint, p. 307; 4. sternoclavicular joint, p. 307; 5. vertebral joints, p. 307; 6. elbow joint, p. 308; 7. wrist joint, p. 309; 8. hip joint, p. 309; 9. sacroiliac joint, p. 309; 10. knee joint, p. 310; 11. ankle joint, p. 311; 12. finger and toe joints, p. 311. 1. Temporomandibular Joint Indications Costen syndrome, temporomandibular joint luxation, temporomandibular joint pain, trismus. Anatomy When the patient opens the mouth wide and closes it, the mandibular condyle slides anteriorly to the zygomatic process, which creates an easily palpable depression. Materials Needle: 20 × 0.4 mm. Quantity 1 mL. Technique The patient needs to keep his or her mouth opened wide. After disinfecting the skin, we insert the needle 10 mm anteriorly to the tragus, toward the side of the nose. The needle is advanced medially into the anterior part of the joint capsule until contact with the bone is made. Now the needle is pulled back slightly and 1 mL of a local anesthetic is injected. 2. Shoulder Joint** Indications Arthritis, humeroscapular periarthritis, capsulitis, stiff shoulder (frozen shoulder), conditions following contusion of the shoulder, arthrosis deformans, subacromial bursitis, before repositioning a shoulder luxation. Anatomy This joint is supplied with its sensory fibers from the axillary nerve (→ (T) brachial plexus), from the → (T) cervical plexus and from the → (T) suprascapular nerve. Materials 0.7 mm diameter 40 mm-long needle. Quantity 2–5 mL. Technique a. The simplest approach is via the axilla, but this is little used because of the proximity of the major vessels and nerves. An accidental injection into the brachial artery, leading as it does to the periphery would not, however, do any harm. Nor should any accidental penetration of the nerves by the needle give rise to any hesitancy. b. Injection from the front: The patient lets his or her arm hang, with the palm to the front. Immediately next to the head of the humerus in a medial direction, it is now possible to feel the articular space. The needle is guided in a dorsolateral direction below the clavicle immediately lateral from the coracoid process. One should remain immediately below the acromion. After overcoming the resistance from the ligaments, it is easy to feel the point of the needle slide readily into the joint (see Fig. 3.12). In many cases it is advisable also to inject into the acromioclavicular joint. Fig. 3.12 Injection into the shoulder joint. c. Injection from behind: The arm is put slightly into abduction and internal rotation. At the lateral end of the scapular spine the angle of the acromion is easily palpable. We insert one fingers’ breadth caudally from there, advance the needle 20 mm in the direction of the coracoid process, and inject 2–5 ml. After the injection into the joint and before withdrawing the needle, we also distribute 1 mL periarticularly. It is advisable to inject about another 1 mm to the periosteum of the anterior inner part of the humerus immediately below the shoulder joint. In cases of fibrosis of the articular capsule or of contractures, the shoulder blade should be fixed and the shoulder joint carefully mobilized directly after the intra-articular injection. A perceptible crepitus indicates stretching of the capsule. It may be advisable to fit an abduction splint and the treatment should be repeated 2 or 3 days later. 3. Acromioclavicular Joint** The lateral clavicular joint creates the connection between the lateral end of the scapula (acromion) and the lateral end of the clavicle. It is a ball-and-socket joint with less mobility than the medial clavicular joint. The majority of arm movements are performed using both joints. Technique After disinfecting the skin, we inject between the lateral end of the clavicle and the acromion vertically from above (with the patient seated). After the capsule resistance ceases, we inject 1 mL. After withdrawing the needle, we inject another 1 mL pericapsularly. (See also → joint diseases and Fig. 3.13). Subdeltoid bursa The subdeltoid bursa (between deltoid muscle and greater tubercle) and the subacromial bursa (between acromion, coracoacromial ligament, and subscapularis muscle) form a unit. In cases of acute and chronic subacromial bursitis we enter through an intracutaneous quaddle two fingers’ breadths laterally to the acromion, at right angles to the skin (in the direction of the contralateral costal arch). At a depth of 20–30 mm we pass through the deltoid muscle. After this resistance ceases, we continue carefully until the point of the needle meets further resistance. This is the supraspinatus tendon. We now withdraw the needle slightly and distribute about 5 mL solution fanwise. 4. Sternoclavicular Joint** The joint between sternum and clavicle is a ball-and-socket joint with three degrees of mobility. This joint allows the movements of the shoulder girdle toward the trunk. It should always be palpated when examining the shoulder joint and treated when sensitive to touch. After disinfecting the skin, we insert the needle from a caudal direction between sternum and clavicle. When the capsule resistance ceases, we inject 0.5–1 mL in 5 mm depth (see Fig. 3.13). 5. Vertebral Joints** The small vertebral and costovertebral joints with their large number of receptors are important control elements of the axial organ and participate in its complicated sequences of motion. Pain as a result of trauma, overexertion, or inflammation can radiate from here (pseudoradicular syndrome) and have a negative effect on adjoining organs. Technique We can eliminate deregulation caused by the small vertebral joints by (after disinfecting the skin) infiltrating with a 50 mm needle vertically, one fingers’ breadth lateral to the spinous process, until we make contact with the bone. We withdraw the needle slightly and after negative aspiration and while maintaining contact to the bone, we distribute the anesthetic fanwise periarticularly. We reach the costotransversal joints by inserting the needle vertically, three fingers’ breadth next to the spinous process, until we reach the bone. At the end of the transverse process we perforate the ligament between thoracic vertebra and rib, which leads us into the joint. We inject 1 mL. See also → lung diseases. Fig. 3.13 The five joints of the shoulder. Right shoulder, anterior view. Fig. 3.14 Injection into the elbow joint. 6. Elbow Joint* Materials Size 12 needle. Quantity 2 mL. Technique The patient lays his or her forearm on the examination couch in such a way that the elbow remains freely accessible from all sides. The forearm should form an approximate right angle with the upper arm. The olecranon and lateral epicondyle are now marked with a felt pen or skin pencil. The entry site is exactly central between these two points. After disinfection of the skin, the needle is advanced about 10 mm in the direction of the antecubital fossa and the procaine is then injected after the articular capsule has been penetrated. Following the injection the joint should be moved, in order to distribute the solution more evenly. (See Fig. 3.14.) Fig. 3.15 Injection into the wrist joint. 7. Wrist Joint* Indications Acute trauma, post-traumatic dysfunctions, before mobilizing a stiff joint. Injections to the stellate ganglion, the parts of the → (T) radial, medial, and ulnar nerves that supply the painful area, and/or intra- and periarterially and around the → brachial artery should be performed, before injecting into the wrist joint or the joints of the fingers. Materials Needle: 20 × 0.4 mm. Quantity 1–2 mL. Technique Injection to the proximal wrist joint: We palpate the joint space between the distal end of the radius and the navicular bone. There, we set an intracutaneous quaddle. After disinfecting the skin, we insert through the quaddle and ligaments, 5– 10 mm deep into the radiocarpal joint and inject 1– 2 mL. With some circulating motions, the patient distributes the injected solution (Fig. 3.15). 8. Hip Joint** Materials 0.8 mm diameter × 80–100 mm-long needle. Quantity 2–5 mL. Technique a. Injection according to Kibler: Skin disinfection! The patient lies on his or her sound side. The sound leg remains extended, the other is flexed slightly, i.e., bent at the hip and the knee. Three fingers’ breadths cranially from the easily palpated trochanter major and past its upper edge the needle is inserted vertically down. When bone contact is made, the point of the needle is at the neck of the femur and within the capsule. It is now withdrawn about a millimeter, and a small amount of synovial fluid is aspirated before the procaine is injected. It should flow out of the syringe without resistance. This injection is completely free of risk, and even if one should accidentally give an intravascular injection, there is nothing to fear below the umbilicus. The effect is often astonishing, but initially it is unfortunately only of short duration. The injection will therefore have to be repeated. Its effect can be increased by an injection to the → (T) trochanter. Individual hyperalgetic points on the skin and in the deeper tissue should be found on each occasion and treated at the same time (Fig. 3.16). b. The patient lies on his or her back, legs together. The needle is inserted vertically down two fingers’ breadths laterally from the pulsating femoral artery on the line connecting the trochanter major and the upper edge of the symphysis until we make bone contact. After withdrawing the needle 1 mm, we inject 5 mL. Now we encourage the patient to walk around to better distribute the solution in the joint (Fig. 3.17). Fig. 3.16 Injection into the hip joint according to Kibler. 9. Sacroiliac Joint** Indications Lumbago, sciatica, static or post-traumatic backache (e.g., due to a shortened leg), non-specific pain in the lumbar-pelvic area (for example, onset of ankylosing spondylitis) also backache in parous women. The sacroiliac joint forms part of the reflex zones of the true pelvis. Most sciatic pains that do not have the classic sciatica symptoms such as those produced by a prolapsed intravertebral disk are due to a blocked sacroiliac joint. Although there is generally freedom of lateral movement in the lumbar vertebrae, forward movement in the region of the lumbar vertebrae is always restricted and the patient often complains of a dragging pain in the area of the sciatic nerve. The joint space is clearly pressure-sensitive. In addition, the sacroiliac joint is almost always blocked or arthrotically changed in coxarthrosis. Fig. 3.17 Injection into the hip joint. 1. From the front: the entry site is two fingers’ breadths laterally from the pulsating femoral artery on a line connecting the symphysis and the upper edge of the trochanter. Go down vertically until bone contact is established, slightly withdraw the needle, then inject. 2. From the side above the trochanter major. 3. Injection to the trochanter major. Materials 0.8 mm diameter × 60–80 mm-long needle. Skin disinfectant. Quantity 2 mL. Technique The patient stands with his or her trunk bent slightly forward. The joint space is found by inserting the needle three fingers’ breadths from the spinous process of S1 and advancing it at an angle of 45° to the skin in the direction of the sacroiliac joint. The neural-therapeutic preparation is distributed at a depth of 30–50 mm. A second injection is then given about two fingers’ breadths in a caudal direction into the irregular articular space and into the interosseous ligament. Thus, in Figure 3.18, the syringe should be moved to the left after entry, to enable the point of the needle to slide past the posterior iliac crest in a lateral direction to the right into the articular space. Since the periosteum of the iliac bone is generally sensitive to pressure at the same time, it is usual to inject a few tenths of a milliliter laterally to the periosteum at the same time. 10. Knee Joint** Indications Arthritis or arthrosis of the knee, recurrent effusions of the knee joint, pain following surgery or dislocation of the knee joint, injuries to the cruciform ligaments or the cartilages (unless surgery is required). Fig. 3.18 Injection into the sacroiliac joint (auxiliary line for orientation). Materials 0.8 mm diameter × 35 mm-long needle. Quantity 2 mL. Technique The patient lies relaxed on his or her back. The knee is slightly bent by being supported on a roll cushion. The patella is now pushed slightly laterally and, after skin disinfection, the needle inserted almost horizontally on the medial edge of the lower third of the patella in such a way that it will slide behind the patella into the joint. If it is guided correctly, the needle will have no resistance to overcome. After the injection the patient is generally immediately capable of pain-free weight-bearing on the knee. The treatment should be repeated after 3– 6 days, later at longer intervals. → (T) quaddles round the knee, including one in the popliteal fossa and especially on the inner aspect of the knee can further increase the effectiveness of this treatment. Another way into the knee is through the popliteal fossa. With this infiltration we also affect the popliteal vein and artery and the tibial nerve. An additional safe technique leads to the suprapatellar bursa that generally fills an indentation in the joint space and, thus, has contact to the joint. After disinfecting the skin, a 40 mm-long needle is inserted two to three fingers’ breadth superior to the proximal edge of the patella. When reaching the bone, the needle is slightly withdrawn and 2 mL are injected into the bursa. If the treatment fails to yield results, neuralgia of the → (T) obturator nerve should be considered a possibility, and the saphenus nerve should be kept in mind. This is the terminal branch of the → (T) femoral nerve, whose infrapatellary branch can produce hyperalgesia above the kneecap and over the medial articular space of the knee if this has been subjected to irritation. In such cases it will be useful to anesthetize these afferent nerves. (See Figs. 3.19, 3.20.) Fig. 3.19 Injection into the knee joint. Fig. 3.20 Injection into the knee joint. Fig. 3.21 Injection from behind into the ankle joint. 11. Ankle Joint* Materials Size 12 needle. Quantity 1–2 mL. Technique a. From behind: Skin disinfection! The surest way is from the fibular side from behind into the upper ankle joint. The patient lies on his or her side in such a way that their foot rests comfortably on its inner aspect. A → (T) quaddle is then set about a fingers’ breadth above the outer ankle bone, i.e., immediately behind the fibular malleolus. The needle is inserted through this, pushed forward horizontally about 10 mm and the injection given into the joint at this point. (See Fig. 3.21.) b. From the front: The patient lies on his or her back. The ankle joint is placed on a small pad and the foot is slightly flexed to allow the tendon of the long extensor muscle of the great toe to appear more prominently. Approximately on the line connecting the two malleoli and immediately medially of the tendon, a slight dip can be felt: the articular space between tibia and talus. The needle is inserted here, immediately next to the tendon, and is guided slightly in and down to slide into the joint. 12. Finger and Toe Joints* Materials Size 16 needle or cartridge syringe with short needle. Quantity 0.3 mL. Technique Injections into the small joints are painful. The neural therapist should therefore always use his or her free hand to fix the finger or toe joint to be treated. This type of injection can be given pain-lessly under a → (T) ring-block anesthetic. As a minimum, however, a → (T) quaddle should be set over the entry point, in order to anesthetize the area, and the needle should then be advanced slowly, infiltrating under steady pressure on the plunger. The joint should be held slightly bent and the injection given from a dorsal direction, but occasionally also from a lateral direction. In the dorsal method, the entry point is immediately to the left or right of the extensor tendon. The point of the needle is then guided slightly forward and down over the head of the bone. Because of the painful tension in the capsule we always limit such injections to a few tenths of a milliliter of procaine or lidocaine. Injection into first metacarpophalangeal joint Before injecting, the patient has to bend his or her thumb back and up as far as possible. This way, the depression of the “snuff box” is formed. It is the depression where snuff is placed and is located directly above the dorsal access to the joint. In order to stretch the joint space, the thumb has to be bend all the way toward the center of the palm. We inject 1 mL. Indications 1. Segmental therapy: Acute or chronic otitis media, otitis externa, deafness of the inner ear, tinnitus and other noises in the ear, vestibular vertigo. In acupuncture, this point is also used for rhinitis and sinusitis; a needle set here immediately eases nasal breathing. 2. Interference-field search: As a test injection, if the patient’s history suggests ear complaints. Contraindications This treatment must not be used in chronic otitis media if there is any cholesteatoma or severe damage to the middle ear, which always require specialist treatment! Materials About a size 12 needle. Quantity 0.5 mL each. Fig. 3.22 Injection to the mastoid process. Fig. 3.23 Injection to the mastoid process. Technique The ear lobe is turned up. We set a → (T) quaddle over the anterior edge of the mastoid process and pass through this down to the periosteum of the mastoid process, giving a few tenths of a milliliter ventrally and dorsally of the mastoid, in order to include the greater auricular and lesser occipital nerves. (See Figs. 3.22, 3.23.) From acupuncture we also adopt a point on the ventral side of the ear, with the suggestive name of “gate of the ear.” This lies in the dimple between tragus and the upper attachment of the external ear. Apart from the otological indications, this point is also used in acupuncture in facial paresis, trigeminal neuralgia, occasionally also for tic and trismus. A further acupuncture point we use is TB-18, about a fingers’ breadth behind the ear, about the middle of the attachment of the ear where we can generally palpate a dimple. An → (T) intra- and paravenous procaine injection into the homolateral antecubital fossa can further increase the effectiveness of this treatment when it is given as segmental therapy. If the ear disorder is segmental, it will be cured if this treatment is repeated often enough. If it is not, every possibility that it might be caused by an interference field elsewhere must be explored. Initially treatment is repeated at weekly intervals and less frequently later. If both sides are affected, they are treated in a single session. The complementary → (T) intravenous injection in such cases will be given alternately left and right; for unilateral symptoms it is given only on the affected side. If there is increased secretion following the injection, this should be regarded as a positive reaction. If there has been a radical operation that has left a deeply indrawn crater-shaped → (T) scar, the treatment should be limited to infiltrating around this scar. Too vigorous an approach in depth is not recommended in such cases, because of the proximity of the meninges. Gross gives a further injection in addition to that to the mastoid, by entering from below the tragus and continuing along the anterior external auditory meatus until bone contact is made, i.e., ventrally to the osseous portion of the external auditory canal. The effectiveness of mastoid anesthesia can sometimes be increased by an injection to the greater auricular nerve. This surfaces approximately at the center of the posterior edge of the sternocleidomastoid muscle and is often found to be hyperalgetic when palpated. If ear scars are present, they are always injected at the same time, and in women patients this must always include pierced ears. I once had a patient suffering from polyarthritis and found a tiny ingrown silver wingnut, which had kept abscesses going over a period of 25 years and finally led to the establishment of an interference field. With the removal of this foreign body, the pathogenic block in the basic autonomic system was eliminated. Small causes, great effects! Anatomy The maxillary tuberosity is a protrusion on the dorsal part of the infratemporal area of the upper jaw, containing three to four foramina for the alveolar nerves. Anesthesia of this tuberosity blocks the upper posterior molars, those branches of the maxillary nerve that supply the maxillary cavity, and the buccal mucosa above the teeth. The maxillary nerve (second trigeminal branch) is a sensory nerve. It passes the foramen rotundum and travels down to the pterygopalatine (sphenomaxillary) fossa where it branches out. It innervates the skin of the maxillary region, the mucosa of the posterior paranasal sinus and palate, and the gum and teeth of the upper jaw. Indications With an injection to the round protuberance at the backside of the maxilla we block those branches of the maxillar nerve (superior, posterior alveolar nerves) that supply the upper posterior molars and the maxillary cavity: 1. Segmental therapy: Disorders affecting the para-nasal sinuses and ethmoid bone. 2. Interference-field search: Test injection when the paranasal sinuses are suspected as an interference field. Materials 0.8 mm diameter 80 mm-long needle. Quantity 1 mL procaine solution. Technique a. Using a spatula or fingers, the cheek is pulled forward. The degree of the acute angle between needle and skin should be as great as possible. The needle is inserted directly behind the bony ridge between the zygomatic bone and the first molar or the anterior edge of the second molar. Applying plunger pressure and maintaining contact to the bone, the needle is advanced posteriorly and superiorly for another 30 mm. Bone contact has to be maintained and, before injecting, aspiration is necessary to prevent injection into the venous pterygoid (sphenoid) plexus. b. A further means of reaching the trunk of the maxillary nerve is the injection to the → (T) pterygopalatine ganglion from the mouth. c. A simple injection to the maxillary nerve: The entry point is half a fingers’ breadth above the anterior quarter of the zygoma. The needle is inserted behind the zygoma and is then directed steeply down toward the center of the floor of the mouth, to a depth of 20 mm, l–2 mL being injected. Anatomy: With the injection into the lower and middle nasal conchae we reach the posterior nasal nerves that originate in the second trigeminal branch, parasympathetic fibers of the → (T) pterygopalatine ganglion, and sympathetic fibers of the → (T) upper cervical ganglion. They travel together to the nasal mucosa. Indications 1. Segmental therapy: Disorders affecting the sinuses and ethmoid. 2. Interference-field search: Whenever there is a suspected interference field in the region of the nose or sinuses. 3. Reflex-zone therapy: Especially for therapy-resistant bronchial asthma, angina pectoris, headaches, and dysmenorrhea. Reflex zones W. Fliess was the first to report about treatment of dysmenorrheal and nervous gastric disorders through anesthesia of the nasal concha. The Frenchman Leprince established that there are four areas of mucosa that act as reflex zones for the following organs: • zone 1: urogenital zone: anterior third of the lower nasal concha; influence on uterus, ovary, ureter, anal and bladder sphincters; • zone 2: solar plexus zone: middle portion of lower nasal concha; influence on stomach, liver, gall-bladder and gut; • zone 3: cervical zone: posterior (i.e., inner) third of lower nasal concha; cervical syndrome, dizziness, tinnitus, anxiety states, migraine; • zone 4: pulmonary zone: anterior portion of middle nasal concha; asthma, pulmonary emphysema. Leprince and others believed that various parts of the human organism could be reached therapeutically from related areas of the nasal mucosa. The theory of ear acupuncture (Nogier) considers that the inverted embryo is represented on the ear and that the entire body can be influenced in a positive sense from certain points of the ear. The theory of iris diagnosis is based on the assumption that the whole organism is reflected in the iris. Reflex-zone therapy, which concentrates on the soles of the feet, originated in South America. All these methods claim good results and tend to assert themselves to be universally applicable. I cannot believe in any rigid blueprint approach to living matter. As far as I am concerned, every living cell makes its contribution to the whole and has stored the plan of the entire organism in the same fashion. Materials 0.8 mm diameter 60–80 mm-long needle. Quantity 0.5 mL. Technique The patient sits on a chair with a neck support. If this is not available, the head must be held firmly! A speculum is inserted in the lower or middle nasal concha and the long thin needle is used to infiltrate the required areas by submucous injections. It is advisable to prepare the patient to expect the nosebleed that ensues and to give him or her a supply of paper tissues. He or she should remain seated for a period afterwards with their head held back. (See Fig. 3.24.) In many cases the → (T) nasal spray described below or insertion of a cottonwool pad soaked in a mucosal anesthetic is likely to prove perfectly adequate and is preferable to an injection. Fig. 3.24 Injection into the nasal conchae: access to the pterygopalatine ganglion from the buccal cavity. Anatomy With a nasal spray we are able to reach the posterior nasal nerves, parasympathetic fibers of the → (T) pterygopalatine ganglion, and sympathetic fibers of the → (T) upper cervical ganglion, which travel together to the nasal mucosa. Materials Procaine cannot be used for anesthesia of the mucosa surface! Instead, 3–6% (!) lidocaine solutions are used, for example, Xylocaine spray. Gingicaine M (tetracaine) and 2% pantocaine must not be used in the case of para-group allergy. Anyone who does not possess a spray of any kind (including a pocket aerosol) can easily improvise with a spray-diffuser. Indications For stopping acute rhinitis and sinusitis, chronic catarrh of the upper respiratory tract, dry irritative cough, silicosis, the start of influenza, certain forms of headache, and vasomotor dysregulatory disorders in the head (for example → cluster headache), dysmenorrhea, hiccoughs; also worth trying in rheumatoid spondylitis (Bechterew disease), asthma and angina pectoris. Also, for mobilizing the body’s own production of ACTH, e.g., in antibiotic-resistant pneumonia. According to F. Huneke, “anesthesia of the nasal and oral mucosa has its own range of indications and can sometimes still lead to success when injection has proved a failure.” Anesthesia of the conjunctiva can alleviate the pain of ciliary neuralgia, and anesthesia of the eardrum can eliminate the onset of otitis media or mild tinnitus. Stubborn dry cough can be helped through surface application of a local anesthetic to the pharynx or the use of an anesthetic spray. Applying spray to the mucosa has an analgetic effect on injection points for testing teeth, eliminates the gag reflex in tonsil injections, removes pain from stomatitis and gingivitis, and allows these inflammations to heal faster. Knothe noted that some of the modern surface anesthetics (for example, Gingicaine and Xylestesin spray) also disinfect the mucosa. Technique We use an anesthetic suitable for the mucosa, such as a 2% Pantocaine solution or Gingicaine, and spray this on the nasal conchae and directly on the upper posterior pharyngeal wall into the vicinity of the pterygopalatine ganglion. Often, simple surface anesthesia of the reflex zones inside the nose, using a cottonwool swab (on a stick) dampened with 2% pantocaine or 1–4% Xylocaine solution (e.g., Xyloneural), is all that is necessary. The swab should be left in place for 3– 5 minutes. Leaving it longer does not increase the anesthetic effect. A local anesthetic can act on a nerve only when it has passed through the nerve sheaths of connective tissue. The nearer a nerve is located to the spinal cord and the thicker it is, the thicker will also be the connective tissue forming the sheath. The more a nerve ramifies and the thinner it becomes, the more readily accessible it is to the anesthetic. The best effect is obtained by → (T) quaddles in the skin, since the nerve fibrils lie completely free in the terminal reticulum. Since the effect is increased the nearer the preparation can be brought to the nerve itself, we try to find the nerve directly. It will then produce its effect more rapidly and reliably, and with the smallest amount of local anesthetic. The pain-conducting C-fibers of the sympathetic system contain the least amount of myelin, are relatively poorly sheathed and are thus the first to be blocked by the local anesthetic, whilst the conductivity of the Aand B-fibers (for touch, pressure, and temperature sensation) continues to function for longer. The perforation of a nerve produces an electric pain and radiates into the area supplied by the nerve, but does not usually persist. If the pain does persist, it disappears within a few days. After we have had to search blindly, the appearance of paresthesia shows us that the needle is in the right place. Warnings have been given against endoneural injections of pharmaceutical preparations, because nerve lesions have been observed. But this does not apply to the use of local anesthetics (without additives!). “For the relief of certain painful syndromes, injections of Novovaine (procaine) can be given into the nerves without hesitation. The risk is so small as to be negligible and need not trouble us” (Leriche). Slow infiltration with constant plunger pressure reduces the amount of fluid that travels in front of the tip of the needle. This lowers the risk that this fluid damages smaller nerves and vessels as it pushes tissue aside. There is one possible complication, however, that must be taken into account: the tip of the needle may become bent due to bone contact and form a barb, which can cause mechanical damage to a nerve when the needle is being withdrawn. We therefore use only sharp disposable needles, which are immediately replaced after brusque bone contact. Though injuries to the nerve cable caused by the needle generally do not produce loss of function, Killian recommends avoiding intraneural injections. Anesthetic deposits should be placed directly next to nerves only. Therapeutic injections into the trigeminal ganglion and the sciatic nerve he lists as exceptions to this rule. The nerve-exit points listed below will be explored over the following pages: 1a. supraorbital nerve, p. 315; 1b. infraorbital nerve, p. 316; 1c. maxillary nerve: see → (T) maxillary tuberosity, p. 313; 1d. mandibular nerve, p. 368; 1e. lingual nerve, p. 316; 1f. palatine nerves, p. 317; 1g. inferior alveolar and mental nerves, p. 317; 1h. occipital nerves, p. 317; 1i. superior laryngeal nerve, p. 318; 1j. glossopharyngeal nerve, p. 318. 1a. Injection to the Lateral Supraorbital Nerve*** Anatomy This is the terminal ramification of the first branch of the trigeminal nerve and forms the frontal and lacrimal nerves of the ophthalmic nerve, which supplies the sensory fibers to the upper eyelid and part of the forehead. Indications Frontal neuralgia, frontal headache, twitching eyelids, heavy eyelids, styes, tarsal cysts, herpes zoster of the first branch of the trigeminal nerve, anosmia. In disorders affecting the frontal sinuses or ethmoid cells, this nerve-exit point is often pressure-sensitive and an anesthetic to this site can act as a healing stimulus on the cavities below; this is shown not infrequently by a heavy, cleansing catarrh. Evidently there is a connection between the supraorbital nerve and the upper abdominal organs. As Ratschow has confirmed, the right supraorbital nerve becomes hyperalgetic in about a third of all gallbladder cases. When this happens, a procaine injection to this point stops all symptoms at a stroke, including colic pains. In gastric disorders we occasionally find the exit point over the left eye pressure-sensitive. Materials Short, medium thick needle (size 12, 14, or 16). Quantity 0.5 mL. Technique By running the lateral edge of the left thumb along the supraorbital ridge, we find the supraorbital notch slightly medially of its center, through which this nerve reaches the surface. The thumb remains in position beside the notch on the inner third of the eyebrow. The needle is inserted with a short thrust in an upward direction in front of the thumbnail. A small arteriole surfaces together with the nerve in this notch and after-bleeding may occur or a hematoma may form. To prevent this, we let the patient apply pressure with a swab for a minute or two to the injection site. If there is no incisure, the nerve might exit through a small hole above the edge of the orbit, as indicated in Fig. 3.25; see also Fig. 3.26. In acupuncture there is a further point in the center of the root of the nose. The two supraorbital points and this third point form the so-called frontal magic triangle, a term that expresses the almost magic effect they produce with regard to the indications given. 1b. Injection to the Infraorbital Nerve** Anatomy The infraorbital nerve originates in the maxillary nerve. Before it reaches and innervates the skin of the anterior parts of the cheek, nostril, lower eyelid, and upper lip including its mucous membrane, it branches off into the superior alveolar nerves that innervate the teeth of the upper jaw and supplies the maxillary sinus where it ends. Fig. 3.25 Injection to the lateral supraorbital nerve. Fig. 3.26 Injection to the supraorbital nerve. Fig. 3.27 Injection to the infraorbital nerve. Fig. 3.28 Injection to the infraorbital nerve. Indications Trigeminal neuralgia, facial pains, furuncles of the cheek, disorders affecting the maxillary sinuses, supramaxillary “rheumatic” pain in the absence of pathological dental findings. Materials Short, medium thick needle (size 12–16). Quantity 0.5 mL. Technique a. Through the skin: If we pass the fingertip along the lower rim of the orbit downward from the inner canthus of the eye, there is at first a smooth section, followed by a slight curve, which runs to the lateral edge. Before we come to this curved section, we feel a roughness. Seven millimeters vertically below this point on the orbit is the infraorbital foramen. We insert the needle slightly below this and guide it obliquely upward in a medial direction until we touch bone. The injection is made there. (See Figs. 3.27, 3.28.) b. From the buccal cavity: Lift the upper lip and insert the needle above the first premolar. The needle is then guided upward to the exit point of the nerve described in (a) above. The needle’s position and progress can be felt through the skin. 1c. Injection to the Maxillary Nerve → (T) Maxillary Tuberosity (p. 313) 1d. Injection to the Mandibular Nerve (p. 368) 1e. Injection to the Lingual Nerve* Anatomy The lingual nerve is a sensory and motor branch of the third trigeminal nerve (mandibular nerve). It travels along the inside of the mandible to the root of the tongue and reaches the bottom of the buccal cavity and the lateral edge of the tongue next to the lower wisdom tooth. It gives sensory supply to the two anterior thirds of the tongue, the internal gum of the anterior mandible, the posterior part of the bottom of the mouth, the tonsillar mucous membrane, and secretory supply to the submandibular gland. The area along the center to the tip of the tongue is supplied by the hypoglossal nerve. Indications Disorders of the tongue, all diseases of the mucous membranes and pain in the area that is supplied, Sjoegren’s syndrome. Materials Cartridge syringes or a needle (size 20). Quantity 1–2 mL. Technique We place a 1–2 mL submucosal deposit at the lingual side of the mandible, between wisdom tooth and tongue. 1f. Injection to the Palatine Nerves* Indications These are given by the area supplied by this nerve: the greater palatine nerve supplies a fan-shaped area forming the greater part of the mucosa of the hard palate (excluding the area of the incisors); the minor palatine nerves supply the mucosa of the soft palate. All nerves contain sensory and secretory fibers. Materials Size 14 or 16 needle or a corresponding needle for a cartridge syringe. Quantity 1 mL procaine solution. Technique These nerves pass out through the greater palatine foramen, which can be identified or felt with a round-headed probe as a small dimple in the mucous membrane medially to the posterior edge of the hindmost molar, on the boundary between the alveolar process and the roof of the palate. We inject 0.5–1 mL of procaine here (see Fig. 3.24). 1g. Injection to the Inferior Alveolar and Mental Nerves* Anatomy The terminal ramification of the third branch of the trigeminal nerve issues through the mental foramen and supplies chin and lower lip. We inject here in trigeminal neuralgia, for pain in chin and lower lip, and for furuncles in this area. If, as described in technique (b) below, we inject perorally to the lingula, we produce conduction anesthesia of the teeth in the corresponding half of the lower jaw. Materials Size 12–16 needle or a longer needle for a cartridge syringe. Quantity 0.5–1 mL for (a); 2 mL for (b). Technique a. Mental nerve: The nerve exit is through the mental foramen, which is found below the lower premolars halfway between the alveolar edge and the lower edge of the mandible. In a toothless jaw, the mental foramen is located on the “pupillary line” (the vertical line through the pupil when looking straight forward), where we find the lateral supraorbital, infraorbital, and mental nerve. Since the foramen runs back laterally, it must be approached at this angle either directly through the skin or from the reflexion in the mandibular mucosa. b. Inferior alveolar nerve: The patient must open his or her mouth wide. To find the entry point for mandibular anesthesia, first feel with the tip of the forefinger back along the buccal side of the teeth until the sharp edge of the anterior border of the ascending mandibular ramus is felt (oblique line). This is the site for starting the injection, i.e., about 10 mm above and buccally from the alveolar margin. The direction of the needle is given by a line connecting this point to the opposite angle of the mouth. The syringe thus lies in the region of the premolars on the opposite side of the mouth. The needle is inserted in a horizontal direction, i.e., parallel with the masticatory surface of the mandibular teeth. After anesthetizing the mucosa and under continual bone contact, we advance slowly another 15–20 mm along the medial side of the body of the mandible and inject about 1.5–2 mL of our solution to the lingula, a small bony projection. This will anesthetize the teeth and gums of the lower jaw, as well as the tongue from the tip to the linea terminalis, because the nearby lingual nerve is anesthetized as well. 1h. Injection to the Occipital Nerves*** Anatomy and indications The dorsal ramus of the second cervical nerve is primarily a sensory nerve. Its strongest branch, the greater occipital nerve, supplies the skin on the back of the head as far as the bregma and to the side as far as the temporal region and the back of the ear. The greater occipital nerve, especially, can be the site of origin of stubborn forms of neuralgia. Sensitivity to pressure at the exit point of the nerve often indicates dysfunction of the paranasal sinus area on the same side of the head (Adler, see → Fig. 1.20 in Part I). We always include the nerve-exit point in the treatment of occipital headache and cervical syndrome. It often produces (through increased blood supply to the → vertebral artery) improvement of memory. This injection can also be helpful in conjunction with treatment of the stressed → thyroid before exams. Materials About a size 12 needle. Quantity 0.5 mL. Technique Before giving this injection, the exit points of these nerves must be accurately located. The greater occipital nerve reaches the surface 20– 40 mm from the midline, between the bony attachments of the trapezius and the semispinalis capitis muscles. It lies directly medially of the easily palpable occipital artery. After aspiration, we inject slowly and only moderate amounts, because some textbooks report the possibility that the local anesthetic travels in a retrograde way from the occipital artery into the internal carotid system. 1i. Injection to the Superior Laryngeal Nerve* Indications Pain and dysphagia, especially in carcinoma or tuberculosis of the larynx, or in neuralgia, hoarseness, whooping cough. Materials 0.8 mm diameter × 60 mm-long needle. Quantity 5 mL. Technique The superior laryngeal nerve divides at the level of the hyoid bone into an external and an internal branch. This is where it can be most easily reached. As a rule, it is desirable to deal with both sides at the same time, so we place a skin → (T) quaddle in the middle over the thyroid notch. We then pass the needle through this quaddle, going subcutaneously under control of the free forefinger in an oblique lateral and cranial direction towards the greater horn of the hyoid bone. Two milliliters of the solution are distributed immmediately adjacent to this in a caudal direction. The needle is withdrawn and the same injection repeated on the other side. This anesthetic reaches the epiglottis and the whole of the upper part of the inside of the larynx as far as the glottis. (See Fig. 3.29.) 1j. Injection to the Glossopharyngeal and Vagus Nerves* Anatomy The cranial nerve IX consists of sensory, motor, and parasympathetic components. It supplies the pharynx, the soft palate, the posterior surface of the tongue, the tonsil, the eustachian tube, and the tympanic cavity. In addition, it also shares in the carotid sinus and carotid body through its secretory fibers: see → (T) Gasserian (otic) ganglion. Fig. 3.29 Injection to the superior laryngeal nerve. The cranial nerve X (vagus) has the same four fiber components as the cranial nerve IX. Together with the glossopharyngeal nerve, it provides motor and sensory supply for the pharynx. It is the only motor nerve for the larynx. It provides partial sensory innervation of the tympanic membrane, parts of the external auditory canal, and the external ear. It is the viscerosensory and parasympathetic nerve for the respiratory tract, beginning at the entrance to the larynx, for the gastrointestinal tract, from the entrance to the esophagus to the left colic flexure, for the kidneys, and the gonads. (See Fig. 3.29.) Indications All disorders within the area supplied by these nerves, such as those affecting the tongue, glossopharyngeal neuralgia, atypical trigeminal neuralgia, dysphagia, cancer of the tonsils and malignancies of the tracheo-bronchial tree, hypertrophic osteoarthropathy (Marie Bamberger syndrome). Materials 0.8 mm diameter × 50–60 mm-long needle. Quantity 2–3 mL. Technique The needle is inserted between the tip of the mastoid and the angle of the mandible, at right angles to the skin until bone contact is made with the styloid process. The local anesthetic is then distributed on the anterior side at a depth of 30– 40 mm. The feeling of a lump in the throat, dysphagia and loss of sensation in the pharyngeal mucosa indicate blocking of the glossopharyngeal nerve. If we infiltrate in a dorsal direction from the styloid process, we anesthetize the vagus nerve. Paralysis of the vocal cords, anesthesia of the base of the tongue, the posterior pharyngeal wall, and of the larynx, together with loss of the tracheal reflexes will persist until the anesthetic effect wears off. Frequently, both these nerves are blocked at the same time. The injection must never be given bilaterally, since in doing so there would be a risk of bilateral paresis of the recurrent nerve. Before giving the injection, the patient has to be informed that anesthesia of the glossopharyngeal nerve also numbs the mucous membranes of the pharynx. This produces the sensation of a lump in the throat and impairs swallowing. The patient needs to remain calm and continue to breathe without trying to swallow the lump. Fig. 3.30 Section of the neck at the level of the cervical plexus. The injections listed below will be covered in the following pages: 2a. injection to the roots of the cervical plexus, p. 319; 2b. injection to the superficial branches, p. 320; 2c. injection to the phrenic nerve, p. 320. 2a. Injection to the Roots of the Cervical Plexus* Anatomy The anterior branches of the segmental nerves C1 through C4 form the cervical plexus. In addition to the diaphragm, its motor fibers supply the subhyoid muscles, the prevertebral muscles, the scalenes, and it has part in the trapezius supply. Its main area of supply is the anterior and lateral area of the neck, up to the area of the ear and the angle of the mandible and down to the second rib and slightly below shoulder level (acromion). The superior part is supplied by the greater auricular, lesser occipital, and cutaneous cervical nerves. The supraclavicular nerve supplies the inferior part. We can affect the superior area mainly by injecting at the third transverse process and the inferior area by injecting at the fourth transverse process. The third transverse process can be found at the angle level with the mandible and the fourth at the level of the upper edge of the thyroid cartilage. Since in the cervical region the intervertebral foramina face forward and not laterally as they do elsewhere, injury to the dura mater and the cervical cord is impossible if the needle enters from the side. If the head is turned sideways, the carotid artery, the internal jugular vein, and the vagus nerve are also displaced laterally and the way to the lateral processes is clear. Indications Cervical syndrome, torticollis, neck pain, burning pain in the shoulder. Fig. 3.31 Injection to the cervical plexus. Materials 40 mm-long needle. Quantity 2 mL. Technique The patient lies on their back, with a roll cushion under their neck. The head is turned to the side opposite to that of the injection. With the fingers of the left hand the sternocleidomastoid muscle is pressed forward and the needle is then inserted at its posterior border at the level of the angle of the mandible. At a depth of no more than 10 mm there is bone contact with the posterior tuberosity of the third lateral process. Without allowing the needle to slide further in, it is guided a few millimeters in a dorsal and caudal direction. Its correct location is indicated by the patient by paresthesia. After ascertaining that neither blood nor liquor is aspirated, inject 2 mL of procaine. The best insurance for avoiding complications is to remain strictly near the surface; in this case at the level of the bone! (See Figs. 3.30, 3.31.) 2b. Injection to the Superficial Branches of the Cervical Plexus** The technically easier injection to the superficial branches of the superficial cervical plexus is preferable to the paravertebral block of the deep cervical plexus described above and, in many cases, this will be perfectly adequate, especially where the deep injection poses problems in patients with a short, stout neck. Anatomy and indications See 2a. Injections to the Roots of the Cervical Plexus. Technique The superficial branches of the cervical plexus surface directly behind the sternocleidomastoid muscle at a point that is called punctum nervosum. We find this point when we draw a line connecting the attachments of the sternocleidomastoid muscle to the mastoid and the clavicle. Bisect this line and insert the needle here, on the posterior edge of the muscle. At a depth of 10–20 mm we inject an area of 20 30 mm with 5 mL of a local anesthetic, while checking constantly through aspiration. 2c. Injection to the Phrenic Nerve* Anatomy The phrenic nerve is the lowest branch of the cervical plexus. Its extrathoracic path runs between the sternocleidomastoid and the anterior scalene muscle. The left-hand phrenic nerve is preferred for this injection. It runs laterally from the aortic arch, pas- ses downward outside the pericardium to the diaphragm. The phrenic nerve transmits organic pain in disorders affecting the cardiac and abdominal regions to the shoulder, neck, and upper arm. Indications Stubborn hiccoughs, severe pain radiating to neck and shoulders in organic abdominal and thoracic disorders; also worth trying in cases of diaphragmatic hernia. Materials 0.6–0.8 mm diameter 40 mm-long needle. Quantity 2–5 mL. Technique Caution: Never anesthetize bilaterally in the same session, because this would lead to total paralysis of the diaphragm! The patient turns his or her head away from the side of the injection and inclines it towards the injection. This relaxes the sternocleidomastoid muscle. The point of entry lies on the lateral edge of the muscle, about 25 mm above its attachment to the clavicle. The sternocleidomastoid muscle is held between thumb and forefinger immediately above the clavicle and is drawn in a medial direction, in order to force the carotid artery away from the phrenic nerve. The anterior scalene muscle can now be felt deeper down. The needle is then advanced under the drawn-away sternocleidomastoid muscle at right angles to the long axis of the body, i.e., almost parallel to the clavicle, obliquely in a medial direction, to a depth of about 30 mm. The finger lying medially on the sternocleidomastoid muscle is used to check the position of the needle as its point penetrates through the scalene notch at a sufficient distance from the trachea and esophagus. The injection is administered only after a negative aspiration test in two directions. The nerves and techniques listed below will be discussed in the following sections: 3a. accessory nerve, p. 320; 3b. suprascapular nerve, p. 321; 3c. brachial plexus, p. 321; 3d. radial nerve, p. 323; 3e. median nerve, p. 323; 3f. ulnar nerve, p. 323. 3a. Injection to the Accessory Nerve* Anatomy The accessory nerve (cranial nerve XI) has motor fibers only for the sternocleidomastoid and trapezius muscle. After exiting the skull, it divides into the internal branch, which turns into the vagus, and the external branch, which is the spinal accessory nerve. It travels to the sternocleidomastoid, which it generally perforates to travel oblique-laterally down through the lateral area of the neck. It arrives at the trapezius muscle. Together with cervical nerves, it supplies the trapezius. Indications Unilateral tonic spasms produce → torticollis, spasmodic. Pain in the trapezius or sternocleidomastoid muscle that does not sufficiently respond to → intramuscular infiltrations. Materials Needle: 40 mm, 0.6–0.8 mm thick. Fig. 3.32 Injection to the accessory nerve. Technique 20 mm caudally to the tip of the mastoid the dorsal edge of the sternocleidomastoid can be palpated. This is the entry point from where we infiltrate the upper part of the sternocleidomastoid with 5 mL of a local anesthetic while slowly advancing the needle. The complete lack of tone indicates the correct placement of the anesthesia. (See Fig. 3.32.) 3b. Injection to the Suprascapular Nerve** Anatomy The suprascapular nerve originates at C5 to C6. It runs under the transverse scapular ligament to the supraspinous fossa. There it divides into its branches, which supply the supraspinatus (abductor of the arm) and the infraspinatus (exterior rotator of the arm) muscles with their motor nerves. Both also have the function of tensioning the capsule of the shoulder joint. The sensory branch supplies the shoulder joint and its immediate vicinity. If the ligament that bridges the suprascapular notch ossifies, this nerve can become irritated, thus causing neuralgic symptoms of obscure etiology in the shoulder region. Indications Vague, difficult-to-localize pains in the thoracic girdle and shoulder joint, suprascapular-notch syndrome, humeroscapular periarthritis; as supplementary injection to any treatment of the shoulder joint. Before reducing a dislocated shoulder, this injection should be given in addition to the intra-articular injection into the shoulder joint, and also to the supraclavicular brachial plexus (→ (T) nerves, see 3c under Injection to the Brachial Plexus) because the subscapular nerve also acts as capsular tensor. It should also be used in recurrent dislocation of the shoulder to improve the tone of the capsular tensors. It merely appears to be a contradiction that we use anesthesia of the suprascapular nerve with recurrent dislocations of the shoulder and with shrinking of the joint capsule. Regulation therapy with local anesthetics balances the function of regulation systems. This can be both tightening and loosening of the joint capsule. Materials 60 mm-long needle. Quantity 3–5 mL. Technique We find the entry point by drawing a line along the spine of the scapula, bisecting this and setting a → (T) quaddle 20 mm cranially and 10 mm laterally from this point. A hyperalgetic point is always to be found here. We pass through the quaddle to a depth of about 20 mm towards the scapular notch until we make bone contact. We then use the point of the needle to probe for the soft tissue of the notch itself. We infiltrate 1–3 mL of our neural-therapeutic preparation here, and a further 1–3 mL is infiltrated laterally to an area about 30 mm wide whilst still maintaining contact with the supraspinous fossa, in order to block this nerve before it divides. If the needle has been sited correctly, the abduction and outer rotation of the upper arm will be put out of action while the anesthetic remains effective, and the patient will be unable to place his or her hand on the back of the head. (See Fig. 3.33.) Fig. 3.33 Injection to the suprascapular nerve. 3c. Injection to the Brachial Plexus C5 to T1** Anatomy The brachial plexus has five different roots of different strength. They travel through the cranial part of the space between the scalenes, along the lateral triangle of the neck, downward between the first rib and the clavicle, and into the armpit, where they form three fascicles around the axillary artery. From the posterior fascicle, the radial and axillary nerve originate; from the lateral fascicle, the musculocutaneous nerve and the lateral roots of the median nerve originate. The medial roots of the median nerve, the ulnar nerve, and both superficial branches of the ulnar nerve of the upper arm and forearm originate from the medial fascicle. Indications All types of plexus neuralgia and brachialgia, painful conditions of the arm (from the distal third of the upper arm) such as paresthesia, circulatory disturbances, post-traumatic osteoporosis, causalgia, phlegmons, abscesses, frostbite or burns in the upper extremities. This injection is also worth trying for writer’s cramp, torticollis, therapy-resistant epicondylitis, humeroscapular periarthritis, and cervical syndrome. Anesthesia of the supraclavicular plexus also facilitates reduction of a dislocated shoulder. Supraclavicular Plexus Anesthesia Materials A thin 40 mm-long needle. Quantity 2 mL. Technique The patient is seated on a chair with a neck support. The head is turned to the side opposite the injection and bent slightly forward. Before the needle enters, he or she must be warned to expect paresthesia, to avoid reflex defensive movements. The entry point is 10 mm above the middle of the clavicle, very close to the pulsating subclavian artery, approximately in the direction of the spinous process of the third thoracic vertebra. At a depth of about 10 mm below this point the supraclavicular plexus passes subfascially over the first rib. (See Fig. 3.34 for the position of the needle in the subclavian injection.) The paresthesia felt by the patient indicates the position of the needle. The nerve pathways from the upper segments lie laterally and those from the lower segments more medially, so that as they are touched from a lateral position in a medial direction the paresthesia will present first in the shoulder, then in the radial side of the upper arm, the forearm, and the hand, further medially in the ulnar side of the hand, the forearm, and upper arm, and finally in the armpit. The radial nerve forming part of this plexus lies in a more posterior position. So, for example, if we first produce paresthesia in the little finger, the needle will be approximately in the middle of the plexus. It is in that case advisable to insert the needle to the left and right of this point and also to infiltrate there. Often, we need to probe with the needle several times here and there to search for the other points where we want to produce further paresthesia. These injections are useless if they fail to produce paresthesia! If the needle reaches the first rib in the absence of twitching in the patient’s arm, this generally means that it is lying too far laterally! We need to stay close to the subclavian artery and go deeper by practically sliding it curvingly along its lateral edge until we reach the first rib. This is a safeguard against penetrating into the pleural cavity and piercing the apex of the lung. We need to note or mark the depth reached where bone contact is made, since we must not go deeper during our careful search for the individual strands of the plexus! If an anesthetic of this plexus is accurately sited, there will be a temporary sensory and motor dysfunction affecting the brachial region. Especially when relatively large quantities of anesthetic are injected, the phrenic nerve may also become temporarily paralyzed; thus, simultaneous bilateral anesthesia of the supraclavicular brachial plexus should not be attempted. There is no risk to the patient if the subclavian artery is punctured. Even if the pleura is punctured, though this can always be avoided if we work carefully according to the procedure described above, it will normally simply produce a tight feeling in the chest, which will persist for a few hours or, very occasionally for as long as 2 or 3 days. Only very few cases of tension pneumothorax have been reported in publications; should this occur, it will, of course, call for in-patient treatment. Fig. 3.34 Anatomy and position of needle in the subclavian injection to the brachial plexus. Axillary Plexus Anesthesia Alternative terminology Axillary block. This method is not so suitable for adipose patients, but it is absolutely foolproof. Palpate along the upper arm along the brachial artery toward the armpit to the point where the pulse is still just perceptible. A total of about 5 mL of procaine is now infiltrated above and below the artery. By this means, it is possible to reach the median, ulnar, radial, and musculocutaneous nerves. The correct location of the needle is shown by paresthesia in the distribution area of the three principal nerves for the arm and hand. Do not inject if no paresthesia is produced! The rapid draining of the anesthetic solution toward the periphery can be avoided by using a tourniquet. Intra-arterial injection of the brachial artery is not dangerous. Larger hematomas are avoided by pressing a cotton pad on the point of insertion for 1 minute. Other Methods See also → (T) stellate ganglion, method according to Dosch, for other means of reaching the upper parts of this plexus from the cervical region. 3d. Injection to the Radial Nerve* Anatomy The radial nerve (C5 to T1) is located dorsally to the axillary artery, in the armpit. From there it spirals laterally around the posterior part of the humerus. In front of the lateral epicondyle it splits into the superficial and the deep branch. Its motor fibers supply the extensors of the upper arm and the forearm, the brachioradialis and the supinator muscle. Its sensory fibers supply the periosteum of radius and ulnar, the radial part of the back of the hand, thumb, index finger, and the radial side of the middle finger, except their distal phalanges. Paralysis of the nerve causes wrist drop. Indications Disorders of the hand in the area supplied by the radial nerve. Technique a. Above the elbow, we can find the radial nerve by setting a → (T) quaddle four fingers’ breadths in a proximal direction of the lateral epicondyle and inserting the needle through this perpendicularly to the skin. At this point the radial nerve runs parallel to the humerus. When the nerve is touched, the patient reports electric pains in the thumb and back of the hand. If we fail to find it immediately, we continue until bone contact is obtained and distribute up to 5 mL up and down along the bone. b. If only the superficial branch is supposed to be affected, feel for the pulse of the radial artery about three fingers’ breadths above the carpal sulcus. The needle is inserted radially of the artery. There would be no danger in an intra-arterial injection of up to 2 mL here. As always, we infiltrate as we advance the needle until the patient reports paresthesia and then deposit 1–2 mL at that point. c. We block the area supplied by the branches of the superficial radial nerve by distributing 1– 2 mL procaine or lidocaine solution in the dorsoradial region of the wrist at snuffbox level. 3e. Injection to the Median Nerve* Anatomy In the neurovascular sheath, the median nerve (C5 to T1) travels downward, along the inside of the upper arm, in the medial bicipital sulcus, which is next to the brachial artery. On the flexor side of the elbow joint, it is located medially to the brachial artery. From there, it crosses in between the two heads of the pronator muscle, remains on the palmar side of the forearm, and reaches the palm through the carpal tunnel. Its motor fibers supply the flexors of the forearm (except the flexor carpi ulnaris and the ulnar part of the flexor digitorum profundus), the pronators of the forearm, the muscles of the thenar eminence (except the adductor pollicis muscle), and the lumbrical muscles. Its sensory fibers innervate the radial part of the wrist and palm, and the first three-and-a-half fingers. Paralysis of the median nerve causes benediction hand. Indications For disorders of the hand (pain, paronychia, whitlow, vasospastic symptoms) in the area supplied by the median nerve; see → carpal tunnel syndrome. Other pressure damage through tourniquets, narcosis paralysis, and paravenous injection can occur. Try in cases of → writer’s cramp. Technique We find the median nerve in the antecubital fossa in an ulnar direction from the pulsating brachial artery. In the region of the wrist two tendons are tensed and clearly visible in volar flexion of the hand: the tendon of the palmaris longus muscle and directly radially to it the one of the flexor carpi radialis muscle. The needle is inserted distally, no more than 20 mm deep, between the two tendons, on the level of the ulnar styloid process, into the carpal tunnel. If the tendon of the palmaris longus muscle is missing (which is the case in 25% of people), the needle is inserted radially to the one existing tendon. As soon as paresthesia occurs in the median region, we infiltrate 1–2 mL of local anesthetic. In patients presenting with a carpal tunnel syndrome, no more than a few tenths of a milliliter (0.1–0.3 mL) should be injected, in order not to exert yet more pressure on a nerve already damaged by compression. In such cases it is preferable to find this nerve further away from the point of constriction, about three fingers’ breadths above the intraarticular space. 3f. Injection to the Ulnar Nerve* Anatomy The ulnar nerve (C8 to T1) perforates the medial intermuscular septum approximately one hand’s breadth proximally to the elbow joint. This brings it from the medial bicipital sulcus to the extensor side of the arm. It is located behind the medial epicondyle, directly between bone and skin. Pressure applied over this point easily causes paresthesia to the area that is supplied by this nerve (funny bone). From here, while covered by the flexor carpi ulnaris muscle, which it supplies, it wraps around to the tensor side. Along this way it sends a muscle branch to the ulnar part of the flexor digitorum profundus, one skin branch to the skin of the hypothenar, and one skin branch to the skin on the back of the hand and its fingers. When proximal to the wrist of the hand, the nerve is located directly next to the tendon of the flexor carpi ulnaris muscle. From there, it travels, superficially to the flexor retinaculum, then to the root of the hypothenar, where it diverges and continues as one skin and one muscle branch. The skin branch supplies palmarly one-and-a-half ulnar fingers, the muscle branch supplies the entire hypothenar, the seven interosseous muscles, both ulnar lumbrical muscles, and the deep thenar muscles (adductor pollicis, deep head of the flexor pollicis brevis). Paralysis of the ulnar nerve causes claw hand. Indications Disorders affecting the area supplied by this nerve (flexor carpi ulnaris muscle, parts of the ulnar digital muscles, and skin areas in the region of the forearm and fingers), complaints of cyclists and motorcycle racers after long rides; see also → scalene syndrome. Technique a. Between the medial humeral epicondyle and the olecranon lies the ulnar sulcus, which can be readily located by palpation. The ulnar nerve can be easily anesthetized in this sulcus, at a depth of 10–20 mm. b. The nerve can be found if the needle is inserted about three fingers’ breadths above the wrist joint between the ulnar artery and the tendon of the ulnar flexor muscle of the wrist, which lies in an ulnar direction from the artery. When we have produced paresthesia, we inject 1–2 mL procaine. The injection techniques listed directly below may be found on the following pages: epidural anesthesia, p. 298; lumbar sympathetic chain, p. 363; sciatic nerve, p. 344; presacral infiltration, p. 334. The techniques listed here may be found in the pages that follow: 4a. femoral nerve, p. 324; 4b. lateral cutaneous femoral nerve, p. 324; 4c. obturator nerve, p. 325; 4d. peripheral nerves in the region of the ankle joint, p. 325; 4e. ring-block anesthesia (fingers and toes), p. 326. Addendum 4f. pudendal nerve, p. 327. 4a. Injection to the Femoral Nerve** Anatomy The femoral nerve (L1 to L4) provides motor supply to the sartorius and quadriceps (knee extensor) muscle. If it is compromised, the patellar tendon reflex is weak and it is hard on the patient to climb stairs. It also disturbs sensitivity on the anterior and medial part of the thigh, the medial area of the knee, and the inside of the lower leg. Indications Neuralgia and paresthesia in the area that it supplies, causalgia, vascular disorders, circulatory disturbances, sports injuries when rapidly over-stretching the hip joint. If the infrapatellar branch of the saphenous nerve (terminal branch of the femoral nerve) is irritated, it may produce hyperalgesia or loss of sensation over the patella or in a more cranial direction from this at the nerve-exit point from the femoral canal, and may also be accompanied by tenderness when pressure is applied over the medial joint space of the knee. Materials Needle: 40 mm long. Quantity 2–5 mL. Technique The technique used for this is described in the section dealing with the → (T) femoral artery, the injection being usually combined. The pulsating artery is found by palpation just below the inguinal ligament, the nerve lying about 10–25 mm laterally from the artery. The patient needs to be prepared that when the needle touches the nerve, paresthesia occurs in the region supplied by it (anterior surface of the thigh), which feels like a light electric shock. Partial anesthetic block of the femoral nerve inhibits motor function of the quadriceps femoris muscle, with corresponding gait disturbances during the anesthesia. This also needs to be communicated to the patient before treatment. 4b. Injection to the Lateral Cutaneous Femoral Nerve* Anatomy The sensory lateral cutaneous femoral nerve is composed of fibers from L2 and L3. It runs from the lumbar plexus via the inside of the iliacus muscle diagonally down and forward. It perforates the inguinal ligament one fingers’ breadth medially to the anterior iliac spine. On the outer aspect of the thigh, some of its branches perforate the fascia lata and supply the skin from the lateral aspect of the thigh as far as the knee. Any toxic or mechanic irritation affecting the cutaneous nerve will produce painful paresthesia accompanied by sensation of soreness, tingling, or stabbing pain on the outer aspect of the thigh. Extension inhibition of the leg due to pain may occur. This disorder is generally known as paresthetic meralgia. Indications Meralgia. Materials 40 mm-long needle. Quantity 2–5 mL. Technique First, we search for the primary pain site through palpation. It is located 10–25 mm in a medial and caudal direction from the anterior superior iliac spine. We place one quaddle at this point. The needle is advanced in the direction of the iliac spine by infiltrating until bone contact is made with the pelvis. Before reaching the bone, the resistance of the fascia lata has to be overcome. The exit point from the fascia is not always in the same place for all patients, and the solution must therefore be distributed subcutaneously and subfascially. This will not produce paresthesia (see Fig. 3.35). Fig. 3.35 Injection to the lateral cutaneous femoral nerve. Fig. 3.36 Injection to the obturator nerve. Anatomical relationships and position of needle. 4c. Injection to the Obturator Nerve** Anatomy The obturator nerve (L2 to L4) reaches the thigh via the upper part of the obturator foramen. It supplies parts of the hip joint, the adductors, the gracilis muscle, and parts of the knee joint. It terminates in the medial cutaneous femoral nerve and provides the sensory fibers to the inside of the thigh. Indications Arthrosis of the hip and knee, neuralgia of the obturator nerve, adductor spasms, gracilis syndrome. Materials 1 mm diameter 80 mm-long needle. Quantity About 5 mL. Technique The patient lies on their back and rotates their thigh outward as far as possible. Palpate in a lateral direction from the symphysis pubis to locate the pubic tubercle and insert the needle about 25 mm caudally of this. The needle is first guided at right angles to the skin until contact is made with the horizontal branch of the pubic bone. It is then withdrawn slightly and advanced further in a lateral direction along the lower edge of the horizontal ramus and introduced into the obturator foramen. The needle will encounter bone at the point where the horizontal branch of the pubic bone turns into the descending branch of the ischium. It is now near the obturator nerve. When this is touched, the patient will indicate paresthesia in the region supplied by this nerve, above all in the anterior part of the hip joint and in the region of the adductor muscles. Following the injection of about 5 mL of solution, temporary paralysis of the adductors may occur and will indicate that the injection has found its mark (see Fig. 3.36). 4d. The Peripheral Nerves in the Region of the Ankle Joint* Indications Following injury, pain, circulatory disturbances, paresthesia, itching, and eczema in the region supplied by these nerves. Materials 40–60 mm-long needle. Quantity 1–5 mL. Anatomy and technique The sole and heel of the foot are supplied mainly by the tibial and sural nerves: a. Tibial nerve: In the tarsal-tunnel syndrome accompanied by pain on the sole of the foot, sensory loss in the toes and in severe cases by hammer toes. The patient lies face down. At the level of the upper part of the medial malleolus, the posterior tibial artery can be felt immediately adjacent the Achilles tendon. We set a → (T) quaddle and insert the needle vertically so that its point will come to lie immediately adjacent and lateral (i.e., dorsal) to the artery, where we inject 2–4 mL. If the tibial nerve needs to be blocked more cranially, it can easily be done in the center of the back of the knee. Fig. 3.37 Injection to (a) tibial and (b) sural nerves. 1. Sural nerve 2. Tibial nerve 3. Posterior tibial artery 4. Small saphenous vein Fig. 3.38 Injection to (c) fibular and (d) saphenous nerves. 1. Saphenous nerve 2. Deep fibular nerve 3. Superficial fibular nerve 4. Great saphenous vein b. Sural nerve: This nerve is formed by the junction of a branch of the tibial nerve with the common fibular nerve; it supplies the heel and the adjacent part of the sole of the foot. It is reached by the subcutaneous infiltration of about 2 mL procaine into the region between the Achilles tendon and the lateral malleolus. c. Superficial fibular nerve: If we wish to treat the dorsum of the foot as far as the toes (with the exception of the lateral half of the big toe and the adjacent half of the second toe, which are innervated by the deep fibular nerve), we locate the superficial fibular nerve subcutaneously, at the level of the ankle joint, between the anterior edge of the tibia and the lateral malleolus. There we distribute about 2 mL. d. Saphenous nerve: This terminal branch of the femoral nerve supplies the area around the medial malleolus and the leg above it. In this case, we again infiltrate only subcutaneously in the area around the great saphenous vein, immediately above the medial malleolus. This is sufficient to block the nerve. After knee surgery, the infra-patellar branch of the saphenous nerve can be irritated. Through infiltration of the scar and repeated injections to the nerve, follow-up neurosurgery can often be avoided. (See Figs. 3.37, 3.38.) 4e. Ring-block Anesthesia of Fingers and Toes*** Indications Paronychia, disorders affecting joints etc. of fingers and toes. Materials About a size 12 needle. Quantity No more than 1 mL on each side. (Caution: vasoconstrictor additives!) Technique Infiltration of finger and toe nerves can be done without problem at the base, injecting 0.5 mm to the digiti dorsales or palmares proprii nerves. The needle is inserted at both sides of the base of the finger, somewhat nearer the extensor aspect. From there, 0.5 mL of a suitable local anesthetic is distributed on the extensor and the same amount on the flexor aspects. The method is the same for the toes, but there, in addition, a third injection site in the middle of the extensor aspect is recommended. (See Fig. 3.38.) Addendum 4f. Injection to the Pudendal Nerve** Anatomy The pudendal nerve (S2 to S4) is the most important sensory nerve of the perineum. After leaving the pelvis through the greater sciatic foramen, it winds around the ischial spine or the sacro-spinal ligament and reaches the perineal region through the lesser sciatic foramen. Together with the internal pudendal vessels, it runs in postero-anterior direction, laterally to the ischiorectal fossa, from which it is separated through the thick fascia of the internal obturator muscle, into Alcock’s canal. It forms the rectal nerves for the skin and muscles of the anus and finally branches off into the muscular branches of the urogenital diaphragm and the muscles of the spongy body, into the perineal branches of the skin of the posterior scrotum or the labia majora, and into the dorsal penile or clitoral nerve. Indications Anal and vulvar pruritus; disorders affecting the scrotum, penis, vulva, and perineum; deep-seated “backache,” coccygodynia and pudendal neuralgia, especially if in vaginal examination the region of the ischial tuberosity is found to be pressure-sensitive. In obstetrics, this injection is also used to relieve pain during the second stage of labor, for vacuum extraction or high forceps delivery, for episiotomy and perineal suture. Materials Needle for (a) 100–120 mm-long × 1 mm diameter, (b) 60–80 mm × 0.8 mm, for (c) PP needle. Quantity From 5–10 mL. Technique a. I have found a way to reach the pudendal nerve, also in a male patient. The needle insertion is identical to the one for the injection to the sacral plexus (see p. 348 and Fig. 3.60). We draw two lines to find it: – The greater trochanter is located one hand’s breadth inferior to the iliac crest, palpable directly under the skin. Three fingers’ breadth cranially and deep to it, we find the upper edge of the bone. From there, we draw a horizontal line to the gluteal fold. – In addition, we draw a vertical line from the lateral depression of the Michaelis rhomboid (superior posterior iliac spine, the protruding, dorsal end of the iliac crest) down to the lateral edge of the ischial tuberosity. The insertion point is located at the intersection of the two lines. Different from the injection to the sacral plexus, we do not advance the needle sagittally, but medially toward the symphysis, until the patient notices paresthesia in the genital area. After negative aspiration test, we inject 5 mL. b. Perineal method: The patient lies on the gynecological couch in the lithotomy position. The ischial spine is found by palpation in a lateral direction either from the vagina or the rectum. The finger is kept in place and guides the needle, which has been introduced transcutaneously adjacent to the vagina (in a male patient at the corresponding location) until it reaches this point. We advance with an 80–100 mm-long needle through a quaddle, guided by the palpating finger, to the ischial spine. Aspiration tests (pudendal artery) are made and the local anesthetic preperiosteally infiltrated around the spine, especially on its lateral and dorsal face. Some of the local anesthetic is then deposited in an anal direction from this and more of it is infiltrated into the ischiorectal fossa, medially from the ischial tuberosity. Injecting in a dorsolateral direction, the sacrospinal ligament is perforated and a further amount of 5 mL is deposited 10 mm beyond this. In this procedure, the posterior cutaneous femoral nerve is also anesthetized. c. Transvaginal method: The patient lies as in (b) above. For this injection it is possible to obtain needles with a protective sleeve. The sleeve enables the needle to be guided directly to the ischial spine. The needle penetrates the mucosa and the ligament immediately adjacent to the spine. After aspiration, 5 mL solution are injected at a depth of 15 mm. (See Fig. 3.39.) Fig. 3.39 Injection to the pudendal nerve. Diagram of perineal and transvaginal methods. Oval foramen See → (T) Gasserian (otic) ganglion and mandibular nerve (p. 368). Para-arterial injection See injections to the → (T) afferent arteries (p. 289). In addition to the injection of the nasal conchae, we also have the possibility of injecting from the mouth to the → (T) maxillary tuberosity and to the maxillary nerve as also described there. From outside we can reach the maxillary sinus either by injections to the exit points of the → (T) supra- and infraorbital nerves or upward to the periosteum of the maxillary reflexion of the mucous membrane at the alveolar margin, and intra-orally through the palate to the floor of the maxillary sinus (palatine nerves, Fig. 3.24). The most important injection is that to the → (T) pterygopalatine ganglion. Paranephral injection See abdominal → (T) sympathetic chain. Parasacral injection → (T) presacral infiltration (p. 334). Paravenous injection → (T) intravenous procaine injection (p. 304). Alternative terminology Nerve block of thoracic or lumbar spinal nerves, paravertebral root block; lower lumbar paravertebral anesthesia to the area of the sciatic root (→ (T) sciatic nerve), according to Reischauer. Anatomy When entering the intervertebral foramen, coming from the spinal canal, the fibers of the anterior motor roots unite with those of the posterior sensory roots, forming in the intervertebral foramen the short trunk of the segmental nerve with (functional part of the posterior root) the spinal ganglion. Liquor space and dura end here. The dura turns into the perineural sheath of the peripheral nerves. Directly at the exit point from the intervertebral foramen, the trunk of the segmental nerve divides into four branches: • the anterior branch for muscles and skin of the anterior and lateral wall of the trunk of the body; • the posterior branch for the autochthonic muscles of the back and one area of skin of the back; • the thin meningeal branch (sinuvertebral nerve), that runs along spinal canal; and • the (sometimes divided) white communicating branch that runs to the sympathetic trunk and sends off the gray communicating branch, which connects with the nerves that travel to the wall of the trunk. Beginning at T12, the anterior branches are part of the formation of the lumborsacral plexus and serve primarily the supply to the legs. If the segmental nerve is temporarily blocked at the point of its exit from the intervertebral foramen, all fibers of the segment are blocked. Indications 1. Diagnostic indications: This injection enables us to block the spinal nerves issuing from the intervertebral foramina, including the sympathetic fibers. This provides us with the means for a differential diagnosis to determine the spinal segment and thus the organ to which the pain reported by the patient should be assigned, and thus enables somatic and autonomic (e.g., cardiac) pain to be diagnosed on a differential basis. Since the level of the spinous process does not necessarily correspond with the level of the segment concerned, it is advisable to refer to Fig. 1.17, Part I on page 70. The main segmental innervation of the abdominal organs is provided by the following: stomach: T7 to T8 left; liver and gallbladder: T9 to T10 right; pancreas: T8 to T10 left; kidney: T12 and L1; ureter: L2 to L4. 2. Therapeutic indications: The therapeutic possibilities of a selective organ-segment therapy are given by what has been stated above. With the paravertebral infiltration we can block the spinal nerves, including the pathological reflexes in the corresponding areas (dermatoma, myotoma, enterotoma), at their exit points. Thus, anesthesia in the region T12 to L1 is used in treating disorders of the kidneys, such as anuria and acute renal failure or oliguria due to spastic or hypertensive causes. In cases of renal or ureteric calculi, anesthesia in the region of L2 to L4 can considerably help the elimination of the calculi! We also use this anesthesia of the spinal nerves for all painful symptoms in the intercostal region, for example, in herpes zoster neuralgia, after fractures of vertebrae or ribs, to relieve pain due to malignancies; also for post-operative pain following abdominal surgery, and for pain in the lumbar, renal, and groin regions, when the diagnosis does not call for other measures. Materials 1 mm diameter × 80–100 mm-long needle. Quantity 5 mL. For paravertebral root anesthesia, it is preferable to use local anesthetics from ampules rather than multiple dose vials, to avoid the preservatives that can cause local irritation, and, if inadvertently injected into an abnormal extension of the dura and subarachnoidal space along the nerve (root diverticuli), can cause cerebral irritation. Technique It needs to be considered that the spinous processes point downward, beginning with the process of the fourth throracic vertebra. From there, the tip of the spinous process corresponds to the level of the nerve segment below. For example, at the level of the spinous process of the ninth thoracic vertebra, we block the tenth nerve segment. In each segment, a → (T) quaddle is set 30– 40 mm laterally of the line formed by the spinous processes. The vertebra prominens and the lower edge of the 11th rib provide a useful surface mark for orientation for this purpose. The spinous process of the seventh thoracic vertebra lies on the line connecting the two tips of the shoulder blades. From the entry point we pass forward exactly in a sagittal direction. To avoid the intercostal artery we keep hard against the upper edge of the rib and the lateral process. After losing bone contact the needle needs to be advanced another 20–25 mm in order to reach the spinal ganglion and its communicating branches. In this region we move the needle back and forth whilst distributing 4–5 mL in each segment. The likelihood of entering the dural cavity in doing so is very remote. Despite this, we aspirate before the injection and whenever we change the position of the needle, especially in a medial direction, in order to make certain that neither blood nor liquor is being drawn into the syringe (Fig. 3.43). If a shooting pain in the foot occurs as the needle is advanced, the nerve root was struck. This has no negative side-effects, and is done intentionally during → (T) sciatic nerve treatment. Possible complications Paravertebral injections in the region of the cervical spine are not without risk and are best avoided. In this area, it is preferable to use the far better injection to the → (T) stellate ganglion according to Dosch. For consequences of meningeal puncture and intrathecal injection, see pages 355. “Pelvic region” is no anatomical terminology. We use it to describe the functional and pathogenetic unit of uterus, adnexa, and bladder. Indications: 1. Segmental therapy: Endo- and parametritis, dyskinesia of the genital organs, dysmenorrhea, menorrhagia and metrorrhagia, non-specific vaginal discharge, cervical catarrh, abdominal pain and backache, autonomic pelvic disorders, sensation of pressure without prolapse; also in constipation and meteorism, dyspareunia, neuritis of the pelvic floor, vomiting in pregnancy, menstruation-related disorders (such as headaches, migraine, mastodynia, exacerbation of skin disorders such as acne or facial dermatitis, abnormal irritability or depressive moods), genital pruritus, sterility, and frigidity, insofar as these disorders are segmental in origin and not due to an interference field. In many cases, we give additional injections into the → (T) thyroid. 2. Interference-field search: As a test injection in extragenital disorders of all kinds, if the patient’s history includes genital disturbances, discharge, abortion, D&C, termination of pregnancy, difficult labor, gonorrhea, pelvic inflammatory disease; also, surgical operations of all kinds involving the external and internal genitals. Even normal delivery can result in an interference field, since enlargement of the uterus leaves histologically ascertainable scars from aseptic necroses. In a surprising number of cases one finds that interference-field disorders originating in the genitals tend to occur after the second labor (Speransky’s second insult or trigger factor). Contraindications None! Menstruation is a physiological process, not a contraindication. Materials 0.8 mm diameter × 60–90 mm-long needle. Quantity Two doses each of 1–2 mL. Technique Preceding the first injection, the patient requires thorough gynecological examination because the technique might have to be adjusted to the palpation findings (for example, prolapse of uterus and vagina, myomatous uterus, adnex tumors such as large cysts, etc.). The routine examination also includes palpation of the posterior symphyseal walls, the entrance points of the → (T) pudendal nerve at the ischial spine, and rolling of an abdominal fold to locate hyperalgesic points of the peritoneum (→ (T) preperitoneal infiltration). Because scars in the segment have to be injected as well, one has to search and inquire about possible impalement traumas and episiotomy scars, vaginal tears (forceps, coital injuries, stupration), surgery scars, vaginal cysts, Bartholin’s abscess, episioperinioplasty, portio surgery, cervical tear, hysterectomy scars, etc. W. Huneke’s technique from 1934 offers a little difficulty only to the beginner; the description makes it seem more complicated than it is in practice! Before the injection, the patient should empty her bladder. She then lies on her back. The point of entry is found about four fingers’ breadths laterally from the symphysis, i.e., about two fingers’ breadths medially from the pulsating femoral artery (and laterally from the inferior superficial epigastric artery) in the region of the upper limits of the pubic hair. If the needle is inserted too far medially, the inferior superficial epigastric artery may be perforated. This may produce an extensive hematoma in the abdominal walls. We press down with the index and middle finger of the left hand until we can feel the upper edge of the pubic ramus under the fingertips. If we now insert the needle between the fingertips at right angles to the skin, we shall reach the edge of the ramus at little depth. This is the position of acupuncture point ST29 at the upper edge of the pubic bone and four fingers’ breadths laterally of the symphysis, with the indications of pelvic inflammatory disease, dysmenorrhea, constipation, meteorism, and ulcer. We inject a small quantity of procaine to this point. The needle is withdrawn slightly and guided cranially past the edge of the bone, and then advanced under steady plunger pressure some 50–60 mm (depending on the adiposity of the patient) in a slightly caudomedial direction. The needle is now lying extraperitoneally close to the reflexion of the pelvic peritoneum. (See Figs. 3.40, 3.41, 3.42.) Fig. 3.40 Injection into the pelvic region. Figs. 3.41, 3.42 Injection into the pelvic region (stages 1 [above] and 2 [below]). From here our injection reaches the upper branches of Frankenhaeuser’s plexus. If one tells the patient to breathe in and out on command, with the mouth open, one can distract her and prevent her from tensing the abdominal muscles with anxiety. There is absolutely no cause for any fear of possible complications. One should also make a habit before this injection to palpate the symphysis and, if it is found pressure-sensitive, to inject there at the same time. Sometimes this injection is made even more effective by another (transvaginal) injection to → (T) Frankenhaeuser’s ganglia or by an intramural injection of the uterus. If the patient develops a marked flush following this injection into the pelvic region, one can be practically certain that an interference field that had previously reduced the peripheral blood supply has been eliminated via a Huneke phenomenon. Periarticular injections See injections to → (T) joints. In neural therapy according to Huneke this method is used only very rarely. It should be reserved for hospital use. However, it is mentioned here for the sake of completeness, since it will enable the practiced neural therapist to use a single injection to reach several segments bilaterally. In fact, in this injection, the anesthetic reaches several pairs of spinal nerves both above and below the injection site as they pass out through the intervertebral foramina. It also acts on the corresponding communicating branches and the ganglia of the sympathetic chain. Alternative terminology → (T) epidural and peridural anesthesia are synonyms. Thoracic or lumbar epidural anesthesia, extradural spinal anesthesia. Anatomy The peridural space is located in the spinal canal. The spinal canal contains primarily the spinal cord, which is covered by several membranes. The dural sheath begins at the foramen magnum. During the growth periods, the dural sheath and spinal cord do not develop to the same size as the vertebral spine. The spinal cord ends at L1 to L3, the dural sheath ends on the level of S2 to S3 (see Fig. 1.19). The space called peridural, extradural, or epidural space, is located between dura and the inner surface of the periosteum. Indications Obstetrics, sciatic-root syndrome, phantom-limb pains, recent frostbite, arterial embolism, post-traumatic osteoporosis, varicose ulcer, lumbago, colic due to calculus, anuria, circulatory disturbances etc. Contraindications Severe circulatory damage. Materials Lumbar-puncture needle with mandrin, a 5 mL syringe filled with physiological saline solution. Quantity 5 mL of local anesthetic (from ampoules not multiple dose vials). Technique First thoroughly disinfect the patient’s skin and allow the disinfectant to act for at least 5 minutes. The patient sits backwards astride a chair and places their arms about an assistant, leaning their head against him or her. As for a lumbar puncture, a → (T) quaddle is first set between two spinous processes in the median plane and the needle is inserted through this. We now aim for the circular space about l mm wide between the two dural layers of the spinal canal, which is filled in the middle with loose interstitial tissue and on the sides by a venous plexus. When the resistance offered by the interspinous ligament has been overcome, the prepared 5 mL syringe filled with physiological saline solution is attached to the needle. The needle is now carefully pushed further forward under constant plunger pressure. In a lumbar puncture, there are two resistant zones that need to be penetrated: the ligamentum flavum and the dura, whilst in peridural anesthesia the needle must stop after overcoming the resistance of the former alone, i.e., before reaching the liquor cavity! When we have reached the tough ligamentum flavum, the resistance this offers is very considerable. To prevent the needle from going too far and into the dura after overcoming this resistance, we need to place our hand flat on the patient’s back and control the shaft of the syringe in such a way that we can immediately stop the sudden jerk that occurs as soon as the needle has passed through the ligamentum flavum. (See Fig. 3.43.) When this ligament has been penetrated, the needle feels as though it were in empty space. As a check, we ask the patient to cough, and aspirate after he or she has done so. If blood or liquor is drawn into the syringe (positive albumen reaction!), the needle is withdrawn and another attempt is made one vertebra higher or lower. Alternatively, the needle is withdrawn until the liquor has barely stopped dripping. On no account must the injection be given into the liquor cavity. When the needle is in the correct position, the syringe containing the anesthetic is attached and its contents of 5 mL injected slowly after a further aspiration test. The appropriate injection sites for various regions are as follows: • upper abdomen: between T6 and T7 or between T7 and T8; • mid-abdomen: between T10 and T11 or between T11 and T12; • lower abdomen: between T12 and L1; • lower extremities: between L2 and L3; • genitals, anus: between L4 and L4 or between L5 and SI. After the injection, the patient should remain lying down for an hour. As a safety measure, it is advisable to check their blood pressure. Should this drop below 90 mm Hg, vasopressor preparations must be injected. Fig. 3.43 Injection into the peridural cavity, showing paravertebral anesthesia and the injection to the sympathetic chain. Perirenal sympathetic-chain anesthesia See abdominal → (T) sympathetic chain (p. Peroneal nerve → (T) peripheral nerves (p. 325). Pharyngeal hypophysis → (T) tonsils. Phrenic nerve See: → (T) nerves (p. 320). Ponndorf’s vaccination is a non-specific irritation therapy. It has proved its worth as a complement and reinforcement of procaine therapy, which, in its effect, is oriented in a similar direction, and its more frequent use can therefore be recommended. Vaccination in the segment can further increase the effectiveness of local therapy by producing additional hyperemia and local relaxation. Such tonus-reversant treatment can cause stubborn chronic conditions to revert to a state where they will again respond to therapeutic measures if it is possible to unblock blocked control circuits and thus to return the reactive response to normal. Ponndorf’s vaccination is one of the means available for this purpose. By a general reversal of tonicity, even desensitization, defensive capabilities are mobilized, which may break through an existing reactive weakness and make the organism again capable of responding to segmental treatment. However, in disorders due to interference fields, Ponndorf’s vaccinations in the segment will not produce any improvement. Indications All forms of muscular and articular rheumatism, particularly primary chronic polyarthritis; arthrosis deformans, gout, neuralgia, neuritis, and general indications for irritation therapy such as asthma, hay fever, dysmenorrhea, chronic mucous-membrane catarrh, chronic eczema and dermatitis, provided that they are not due to an interference field. Contraindications Tuberculosis, cachexia, severe renal disease. Materials Special vaccination fork or a normal vaccination lancet; the point of a needle or an ampoule file will also do. Koch’s old tuberculin or Cutivaccine Paul Novum. Technique We use undiluted Koch’s old tuberculin or Cutivaccine Paul Novum. The latter consists of an autolysate of bacterium subtilis with the addition of a mixture of tuberculin and glycerine. Over particularly painful joints or areas of skin or muscle, the skin in the segment is scratched quite superficially with the vaccination instrument or the point of an ampoule file, as for a smallpox vaccination, i.e., only in the epithelium and without causing any bleeding, so that the capillary blood can just be seen showing through. We make four or five scratches over the chosen area, 10–20 mm apart and about 50 mm long, and rub in one to five drops of undiluted old tuberculin or Cutivaccine Paul Novum until dry. No bandage is necessary, but if required some gauze or cellulose wadding may be placed over the vaccination site and secured with adhesive plaster. The vaccination site must not be washed for 2 or 3 days! The vaccination is repeated at intervals of 1–2 weeks, depending on the reaction produced, and of 4 weeks at a later stage, for a total of from five to 10 vaccinations. If the reaction is negative, the vaccination area may be increased when the treatment is repeated. The symptoms of this provocation can be anything from slight itching to pain ranging from slight to severe, and may include urticaria and skin inflammation. There may be a general reaction, such as feeling unwell, headaches; more rarely, shivering and fever. But these disappear without further treatment within 24 hours (if necessary, an analgesic may be given). The more severe the reaction, the better the effect. Blisters and papule formation, which may produce superficial necroses, are extremely rare. If there is a severe reaction, first wait for the symptoms to disappear before repeating treatment, and in that event the area of skin subjected to the vaccination should not be increased but possibly even reduced somewhat. The second vaccination generally produces the strongest reaction. The patient should be warned that this treatment may activate an existing interference field or focus. They should make a note of any scars, teeth, appendix, or other organ, which produce symptoms as a result of this provocation and report these before the next treatment session. Obviously, any interference field that announces its presence in this way must be tested and treated until it has been eliminated. If the basic disorder is exacerbated after the vaccination, even if there is no local reaction, the suspicion is justified that the cause lies in an interference field or focus. If there is no reaction, this does not prove that there is no focus or interference field. As stated in Chapter 8, Part I, Section A, Interstitial Connective Tissue and Interference Fields, regulatory paralysis may prevent the organism from responding to such provocation. Baunscheidt’s vaccination and the use of cantharides have often rendered us equally good services. Posterior sacral foramina See posterior → (T) sacral foramina. Anatomy The periosteum is a tight connective tissue membrane that covers the bone (except the joint part covered with cartilage). It is rich in nerve and blood vessels. Its inside contains osteoblasts, which are cells involved in bone formation. The neural tissue of the periosteum has many receptors that can affect the corresponding segment and the vegetative regulation through osteovisceral reflex pathways. This can be influenced through periosteum massage (Vogler, Krauss) and procaine or lidocaine injections to (not under!) the periosteum. Materials The length of the needle depends on the depth of the intended periosteum injection, i.e., 20– 60 mm long. Quantity 1–5 mL of a local anesthetic. Indications Pain of the periosteum can be the result of mechanical muscle overexertion and indicate a chronic inflammatory irritation. It can also indicate disturbances within the segment. Every irritation of the periosteum can produce pseudoradicular pain: 1. Segmental therapy: Perisostitis, periostoses, osteomyelitis, fractures, Sudeck’s dystrophy, headache, ear disorders, spinal pain such as cervical, thoracic, lumbar spine syndrome, angina pectoris, intercostal neuralgias, coxalgias, and many more. 2. Interference-field search: Conditions following periostitis and fractures, which are not pain-free, osteomyelitis, etc. If we find pressure-sensitive points on the periosteum during palpation, they have to be anesthetized with preperiosteal infiltrations. This way, we can interrupt pathogenic interactions between periphery and underlying organs. Periphery and organs are connected not only through blood circulation but also through neural and humoral pathways which can be used to initiate functional and reactive changes in underlying organs. This has been confirmed by experiments. On the head, particularly the temples, the occiput, the mastoid, and the atlas transverse processes can be painful (see Fig. 1.20, Part I). Clavicle, sternum, and ribs have to be palpated as well. In cardiac and lung diseases, hyperalgesic points can be found mainly on the anterior thorax and lateral, in the armpits. Along the spine, such points on transverse and spinous processes can indicate visceroperiosteal disturbances. Muscle groups that are constantly tightened due to reflex action will show painful responses on their periosteal attachments. In all joint pains, not only the periosteum around the joint should be examined, but also muscles and tendon attachments that are close by. In abdominal diseases, Vogler and Krauss found indentations, roughness, deposits, and similar changes at the inferior edge of the thorax. Through periosteum massage they were able to produce objectively verifiable improvement of the diseased abdominal organs. In diseases of the hip joint, the periosteum of the trochanter major is often pressure-sensitive. The iliac crest, the sacrum, the coccyx, and the symphysis have to be palpated as well. Distal disturbances can be caused anywhere and we have to find and reduce them. Through the change of local and general sensitivity, the irritation threshold is raised and functional balance reinstated. Technique After a → (T) quaddle is set above the pressure-sensitive point, the needle is advanced down to, not under, the periosteum. Subperiosteal injections can cause intense pain for a few days after the injection. Theoretically speaking, subperiosteal infiltration offers no advantage over preperiosteal infiltration, because the periosteum receives tropic and sensory supply from the soft tissue that is located above it. However, the pain stimulus following subperiosteal injections can produce additional responses. For example, considerable improvement of epicondylitis pain is reported after the strong response subsides (irritation therapy). In treatments of the periosteum, the therapeutic effects last considerably longer than the actual anesthesia. If the pain returns, infiltrations have to be repeated. The effect increases with every repetition. If there is no improvement, other segmental injections have to be tried or an interference field has to be located. Anatomy The perietal peritoneum covers the walls of the abdominal and pelvic cavities with a serous membrane. Abdominal organs do not possess sensitive mechanosensory innervation. Pain transmission takes place via vegetative fibers of the splanchnic nerves and the celiac ganglia or other corresponding ganglia and nerves. The peritoneum receives sensory innervation from the nerves of the truncal wall. The pain-conducting nerve fibers of the organs in the peritoneal cavity run together with the sympathetic fibers of those organs. The conducted pain manifests itself in the Head’s zones. The highly sensitive parietal peritoneum can show signs of intra-abdominal regulation disorders and inflammations, which we can locate through palpation and feedback from the patient. Due to positive reactions following our injections to hyperalgesic points in the abdominal wall, we can conclude that we can interrupt pathogenic feedback of visceroperitoneal and peritoneovisceral pathways through anesthesia. After proper repetition, the feedback can be regulated. The list of indications includes all inflammatory and autonomic dysregulation of abdominal organs, also mucous and ulcerous colitis, diverticulitis, frigidity, etc. Contraindications All acute surgical indications, for example acute → appendicitis and conditions without sufficient differential diagnostic findings. Materials Needles have to be chosen according to the thickness of the abdominal wall. Quantity 2–5 mL. Technique The patient lies supine with a pillow under their knees to relax the abdominal muscles. After careful inspection and palpation, we have the patient mark skin over the center of discomfort with their fingernail. We set a → (T) quaddle at the nail marking. Now we grab the abdominal fold above the upper pubic crest between thumbs and fingers of both hands and test thickness and pressure sensitivity by rolling the fold slowly cranially (see Fig. 3.44). In the case of abdominal dysregulation, this will cause pain of individual intensity, which originates in the abdominal walls and the irritation reflex of the peritoneum. We set quaddles above all palpable hyperalgesic points. We advance slowly, deeper, and search carefully in a fan-shaped area. When the patient reports acute pain in a closely circumscribed area, we have reached the correct location and deposit 1 mL. We also search for hyperalgesic points in the dorsal Head’s zones of the lumbar and sacral area. Those are treated as well. If that is not enough, we will include other injection techniques that apply to the segment, for example, into the → (T) pelvic region, → (T) paravertebral infiltration, and injection to the → (T) sympathetic chain and its → (T) ganglia. As always: search for an interference field. Fig. 3.44 Rolling of the abdominal fold. Alternative terminology Parasacral anesthesia (Braun, Pendl). Anatomy The sacral nerves S1 through S4 issue from the vertebral foramina of the sacrum and form part of the lumbosacral plexus. They also provide parts of the pudendal plexus. When we block the sacral nerves and infiltrate around the coccyx and in an anterior direction next to the anus and perineum, we anesthetize the anus, anal sphincter, rectum, perineum, urethra, and bladder. Further, in men, the penis, scrotum, and prostate are also anesthetized, whilst in women it is the vaginal introitus and vagina, the uterine cervix, the pelvic floor, the parametria, and part of the pelvic peritoneum. According to Killian, the presacral infiltration is part of the paravertebral anesthetic blocks. Indications The indications are given by the anatomy: disorders involving the rectum, anus, perineum, bladder, and urethra; pruritus of anus and vulva, gynecological disorders, prostate, constipation due to rectal atony, sciatica, intermittent claudication, varicose ulcer, and other circulatory disturbances affecting the lower extremities, lumbago with non-specific origin. Materials l mm diameter × 120–150 mm-long needle. Quantity 2 × 5 mL. In anesthesia for surgery, quantities from 100–200 mL of 0.5% procaine solution are used. For neural-therapeutic purposes, 5 mL to each side suffice to produce the stimulus we seek to provoke, but this amount needs to be well distributed to the individual sacral foramina. Technique The knee-elbow position is felt by some patients to be indecent; it is not absolutely essential for this injection. It will suffice for the patient to stand hard up against a table, bend their trunk forward at right angles and place it on the table. To avoid damage to the intestines, the neural therapist with little practice in giving this injection should introduce the gloved and lubricated left forefinger into the patient’s rectum in order to control the needle; with a little practice this precaution can be omitted. Fig. 3.45 Presacral infiltration according to Pendl (topography). Fig. 3.46 Presacral infiltration according to Pendl. The needle is inserted a finger’s breadth laterally of and below the tip of the coccyx. It should be at least 120 mm long and is guided in a cranial direction on the ventral side of the sacrum. There is no risk of injury to the rectum if the needle is advanced under constant plunger pressure almost parallel to the median plane up along the anterior face of the sacrum and remains in bone contact with the sacrum. The needle is advanced and withdrawn in accordance with the curvature of the sacrum, infiltrating all the time, in order to distribute the anesthetic and let it spread evenly over the area of the sacral foramina. The uppermost of these lies about 100–120 mm from the entry site, the second about 80–90 mm distant. Before the needle is finally withdrawn, we also give a few tenths of a milliliter laterally below the coccyx, in order to include the coccygeal nerves. This injection can, of course, be administered without risk bilaterally in a single session. (See Figs. 3.45, 3.46.) Anatomy In men, the pelvic plexus supplies anus, urinary bladder, and genitals through the vesical, deferential, and prostatic plexi and the cavernous nerves of the penis. Bradley showed that the prostate capsule (in women the vesical trigone at the base of the bladder) contains receptors that generate afferent sensory impulses. They regulate the Barrington reflex arch between muscles that allow the bladder to fill and empty. In order to regulate disturbances that originate at this point, injection to the prostate capsule is sufficient. Indications 1. Segmental therapy: Adenoma of the prostate, acute or chronic prostatitis (prostatism), non-bacterial prostatopathy, micturition problems (such as dysuria, nocturia, pollacisuria, urge incontinence), therapy-resistant anal pruritus, proctitis; also to be tried in sexual disorders and irritable bladder, for example, after extensive transurethral resection up to the capsular area. 2. Interference-field search: As test injection, if the patient’s history includes gonorrhea, prostatitis, epididymitis, non-specific urethritis, and the like, but especially if he has to get up several times a night to urinate or reports corresponding micturition difficulties. In geriatrics, patients often report spontaneously that after procaine treatment of the prostate they feel younger and more efficient, and that rheumatic symptoms, coronary spasms, and other secondary symptoms have disappeared following treatment. Contraindications Carcinoma in the area of the urinary tract, tuberculoses of the genitourinary tracts, inflammatory, degenerative, and traumatic disorders of the central regulating mechanisms of the central nervous system, and diseases of psychological origin. Technique a. The perineal approach: The patient strips below the waist and is placed on a gynecological examination couch. With one hand he holds up the scrotum. The physician introduces the gloved and lubricated forefinger of the left hand into the patient’s rectum. The fingertip should press the prostate slightly forward towards the needle, which is inserted through the perineum about 10 mm one side of the midline and 15 mm cranially of the anus, and is then guided under control of the physician’s finger in the patient’s rectum to bring it directly into the prostate (Fig. 3.47). Injury to the rectum must be avoided at all costs. If the needle is inserted directly above the anal sphincter (i.e., not too far cranially), perforation of the cavernous body can be avoided with certainty. Fig. 3.47 Injection into the prostate. The needle is guided from the perineum directly into the prostate, under digital control from the rectum. Fig. 3.48 Suprapubic injection into the prostate. When the point of the needle is in position in the gland, 1–2 mL are infiltrated, and the same is repeated on the other side. If the gland is greatly enlarged and hard, it may offer considerable resistance to the anesthetic flowing in. If the plunger is released after the injection, it will often return to its original position. This means that practically the whole of the injected material would run out of the injection canal if the needle is withdrawn too quickly. It is therefore advisable to wait a few seconds after giving the injection before withdrawing the needle, and to distribute the injected liquid in the gland by massaging it with the forefinger in the rectum, until there is no more back pressure on the plunger. Before withdrawing the needle completely, we inject another milliliter outside the prostatic capsule. A brief prostatic massage is in any event to be recommended, since it helps to distribute the material more thoroughly throughout the gland. Some urologists have voiced concerns about our injections into the glandular tissue. They are worried about the spreading of tumor cells. After decades of experience, we cannot confirm this concern. First, we never inject into hard glands with a bumpy surface that could be cancerous! Also, instead of 2 mm diameter Trucut needles, that can cause complications, we only use needles of 0.8 mm diameter that correspond to the fine needles used in biopsy. Inoculation metastases have been found in pancreas carcinoma with lowered immune response after biopsy with Trucut needles. Literature lists spreading of tumor cells after fine needle biopsy as “extremely rare occurrences” of 1:20 000. The patient should be warned before this treatment that he may have a certain amount of bleeding from the urethra; this is harmless and generally requires no further treatment. At most, an oral hemostatic preparation suffices. The seminal fluid may also be bloodstained at the next ejaculation. The injection should be repeated after about a week, and once the symptoms begin to disappear the intervals may be increased. Segmental treatment of the prostate must not be stopped too soon if the results obtained are to last. b. The retropubic approach: If for anatomical reasons such as ankylosis of the hip an injection into the prostate is not possible, we inject (as in the injection into the → (T) pelvic region) to the upper edge of the pubic ramus and then deeper in a mediocaudal direction towards the prostate. When the point of the needle enters the gland, the patient reports a pain radiating to the glans penis. If one makes use of the alternative retropubic technique by introducing the needle behind the center of the symphysis and guiding it parallel to the posterior wall of the symphysis in a caudal direction, it will reach the middle lobe of the prostate. In older patients, this often hypertrophies on its own. The different prostate injections may also be given alternately and one then asks the patient to report on which has been the most effective in his particular case. In patients who have undergone prostatectomy, an injection into the prostatic bed will show whether this deep surgical scar is acting as an interference field. Quaddle therapy is the best-known and most practiced form of neural therapy. It is quite effective, but usually only the initial treatment followed by additional injection techniques. If the therapist is content with this option and its results only, he or she misses greater success that results from the advanced techniques. Nobody drives a car in first gear only! The skin is the human being’s largest and functionally the most versatile organ. “There is no disorder which can be cured without the skin playing its part” (Hufeland). Tracing back human development, epidermis, nervous system, and sensory organs originate in the ectoderm. This could explain why drugs acting on the nervous system have such an affect on these systems. According to W. Scheidt, in addition to vascular and connective components, about 90% of all autonomic nerve substance is in the skin! This wealth of sensory and autonomic nerve fibers and receptors on the body’s surface gives the skin a place of special importance, and we make use of this in the segmental treatment of both healthy and pathologically changed skin. Anesthesia of the skin through quaddles blocks the pain receptors that transform all excessive mechanical, thermal, and chemical stimulations into electrical signals. This way, information about pain and other morbific stimuli that travels to the posterior horn and from there to the central nervous system, can be interrupted and later reduced. Stimuli that originate in the skin can travel through cutanovisceral pathways and produce visceral pain, dyskinesia, and secretory disorders. On the other hand, warming of the skin can calm the organs of the corresponding segment and improve their blood supply. The interaction and interdependence of the internal organs and the skin are ancient empirical knowledge in medicine, and are utilized in a number of forms, such as acupuncture, skin-irritation therapy, the application of heat or cold etc. (see Fig. 1.11, Part I). An intracutaneous quaddle is incredibly effective as regards both the extent and depth of its action. Thus, its selective, pinpointed use forms a substantial part of our therapeutic armory. Near their terminal branches, the nerve fibrils no longer have any sheaths of connective tissue. As a result, the local anesthetic can act directly on the nerve endings and bulbs to repolarize them without first having to overcome any resistance. Quaddles are a special form of irritation therapy. They act as a stimulus that can reverse autonomic functions via segmental reflex channels in areas related to the segment. Further, by the autonomic interaction of the control circuits and systems, a response can be produced in all the related substrates and tissues. Table 1.1 in Part I shows dermatomes that correspond to organs for quaddle therapy. Every intracutaneous injection sets off a large number of non-specific general reactions that are quite independent of the site of the injection. So, for example, the following have been found to occur: • a drop of about 26% in the leukocyte level after about 10 minutes; • reduced blood pressure; • increased acid excretion in the urine; • increased capillary permeability in the vicinity of a quaddle, with an escape of colloidal blood particles that do not normally reach the skin and that produce a substantial effect on important humoral regulating complexes. Apart from this general effect, there is the specific action that is produced by selective, pinpointed injections to particularly effective points and in disturbed segmental tissue. The injection of a local anesthetic to these points can further increase the effect. Naturally, we can only give suggestions but no generally valid points or zones for every “diagnosis.” The analgesic points that require treatment have to be located individually through extensive exploration and palpation of the indicated area and its surroundings. The general points listed in the text have to always be complimented by the individual points of the patient. The techniques listed below will be covered in the following pages: 1. scalp p. 338; 2. mastoid process, p. 338; 3. parasternal quaddles, p. 339; 4. bilateral quaddles to the thoracic spine, p. 339; 5. quaddles over the epigastrium, p. 339; 6. quaddles in the pelvic region, p. 339; 7. quaddles in the sacral region, p. 339; 8. quaddles over diseased joints, p. 339; 9. quaddles on thigh and leg, p. 340; 10. quaddles in diseased skin, p. 340. Refer also to the figures dealing with Head’s zones (Figs. 1.16, 1.17). Alternative terminology Intradermal or intracutaneous anesthesia. Materials About a size 20 needle (special quaddle needles are not necessary). Quantity 0.2–0.4 mL per quaddle. Technique A quaddle should be strictly intracutaneous (see Fig. 3.49). In this form it is far more effective than a subcutaneous injection. The fine needle is inserted flat, almost parallel to the skin, until its opening, which should always face up, just disappears below the epidermis. When the plunger is pressed, a pale, circumscribed swelling like an insect bite will form, whose surface is reminiscent of orange skin. This swelling is termed “quaddle.” The effectiveness of a quaddle may be further increased by injecting air. The syringe is not completely filled with procaine and is held with the point up. If the plunger is then withdrawn as far as it will go, air will be drawn in. The syringe is held with the point up as the needle enters the skin. As pressure is applied to the plunger, this air must escape first. It will separate the skin and inflate it suddenly over an area up to 30 mm in diameter. As the procaine flows out, it can spread more easily and over a larger area. Such combined emphysema-procaine quaddles are used with particularly good effect for heart and lung conditions, when they are given parasternally and/or bilaterally next to the spine, in the vicinity of varicose ulcers and for injections to Head’s zones. If a procaine quaddle is set within an area of cyanosed skin, for example, in the vicinity of an earlier varicose ulcer, where the over-saturation of the blood with carbonic acid as a result of venous congestion is clearly visible, a bright red zone about 10 mm in diameter will quickly appear. After a procaine-and-air quaddle, the diameter of this zone will be at least 30 mm. There is no doubt that the quaddle has a deep-acting effect that is adequate in a large number of cases. We can see this, for example, in cases of lumbago, where the patient will happily report an improvement of about 80% after a few correctly sited quaddles. To make absolutely certain, it is common practice amongst neural therapists to pass through the quaddle and inject directly into the hyperalgetic tissue at the required depth. Before injection, whatever the symptoms or disorder, in addition to quaddling the well-known and frequently recurring “regulation zones” it is always necessary to find the patient’s “personal” hyperalgetic zones and points relevant to each particular case, and to quaddle these also. The success or failure of any quaddle therapy depends decisively on the correct siting of the quaddles and thus on the prior examination of the patient. Any bleeding that occurs is stilled by pressure with a cottonwool swab. Fig. 3.49 1. Intracutaneous quaddle 2. Injection through a quaddle into a fibrositic nodule 1. Scalp At the level of the parietal bone or in the temporal area, intra- and subcutaneously down to the periosteum below, always in conjunction with an → (T) intravenous procaine injection, for: alopecia, headaches, insomnia, dizziness, cerebral contusion or concussion and its sequelae, traumatic epilepsy (plus all head scars down to the periosteum!), for presclerotic or spastic cerebral circulatory disturbances, paralysis agitans (Parkinson disease), pre-and post-apoplectic conditions, diabetes insipidus (see Figs. 3.54–3.56). Over the mastoid process in conjunction with injections down to the periosteum and an → (T) intravenous injection, for disorders of the ear, such as acute or chronic otitis media (but not if there is a cholesteatoma or severe damage to the middle ear, which must be treated by a specialist), mastoiditis, deafness of the inner ear, vestibular loss of equilibrium (see Fig. 3.22). 3. Parasternal Quaddles One quaddle to each side of the upper sternum, and a third over the angle formed by the xiphoid process with the left lowest rib. Women frequently also report a hyperalgetic point under the attachment of the left breast, and an additional quaddle should also be set here. If necessary, one may go through this quaddle to the inferior edge of the rib to the periosteum, the inter-costal nerves or as far as the pleura. In esophagus disorders, bilateral quaddles to the edge of the sternum, above the intercostal spaces, in addition, bilaterally to the corresponding area of the thoracic spine (T5 to T8); also injections into the epigastrium. In cardiac patients, we give an additional → (T) intravenous injection on the left side, in lung patients alternately left and right, and also, in both these cases, the quaddles described in (4) below next to the spine (see Fig. 2.7, Part II and Table 1.1, Part I). 4. Bilateral Quaddles to the Thoracic Spine In addition to the parasternal quaddles described in (3) above and the → (T) intravenous injection, we set five or six quaddles on each side over the shoulder and two to three fingers’ breadths from the interspinal line, for lung disorders such as asthma, silicosis, whooping cough; sequelae of bronchitis, pneumonia, pleurisy, pulmonary tuberculosis. This tests the “thoracic area” for a potential interference field (see Fig. 2.8, Part II). 5. Quaddles over the Epigastrium The injection is administered with the patient lying on their back. We set a quaddle over the celiac ganglion, three fingers’ breadths below the xiphoid process. We then pass through this quaddle and go deeper to the peritoneum of the → (T) epigastrium. Here again, we set additional quaddles over hyperalgetic points found by palpation, e.g., over the gallbladder, the edge of the liver, the pylorus, Vogler’s points on the inferior edge of the thorax, and in the corresponding Head’s zones on the shoulders and between the shoulder blades. If this treatment in the upper abdominal region does not suffice, we combine it with an injection to the abdominal → (T) sympathetic chain (see Fig. 3.10). Fig. 3.50 Quaddles to the pelvic region. 6. Quaddles in the Pelvic Region We distribute about four quaddles over the ventral Head’s zones of the pelvic region, i.e., over the area of the bladder and the mons pubis, for gynecological disorders such as vaginal discharge, dysmenorrhea, endoand parametritis, pelvic inflammatory disease, menoand metrorrhagia, sterility, lack of libido, and disorders affecting the urogenital tract, e.g., inflammatory, functional, and dystrophic symptoms of the bladder, prostate, and renal pelvis. Generally, we also set the quaddles over the sacrum as described in (7) below, possibly in conjunction with injections into the → (T) pelvic region or to → (T) Frankenhaeuser’s ganglia. (See Fig. 3.50.) 7. Quaddles in the Sacral Region Over the sacrum we set six quaddles in the dorsal lower abdominal Head’s zones. The highest pair of these lie over the lateral dimples of Michaelis’s rhomboid, the lowest directly next to the upper limit of the natal cleft, about 20 mm apart. The four upper quaddles cover the area innervated by the hypogastric plexus; the lower pair act on the outer anal and vaginal zone S4 to S5 (see Fig. 3.51). 8. Quaddles over Diseased Joints Over diseased joints, especially the shoulders, elbows, knees, and ankles, we set a series of quaddles for arthrosis, arthritis, bursitis, tendovaginitis, sports, and other injuries with hematoma, sprains, torn ligaments, and fractures. Infiltrations may also be given fanwise through the quaddles into the painful zones and in depth to the main hyperalgetic points. Fig. 3.51 Quaddles to the sacral region. In the case of the knee, 2–3 mL of procaine are distributed in five or six quaddles all round the joint, as follows: • on the outside, one over the joint line; • on the inside, one each over the head of the tibia, over the joint line, and over the head of the femur, these three forming a triangle (Fig. 3.52) in the center of the popliteal fossa (acupuncture point for skin disease, paresis of the lower extremities, arthrosis of the knee and sciatica). If no satisfactory result is obtained, it is possible to pass through the last of these quaddles to infiltrate at depth to the blood vessels, the nerves, and to and into the joint capsule, in order to increase the effectiveness of the ring of quaddles. If other painful points are found in the course of palpating the affected joint, these will obviously deserve priority treatment. The extensive effects of quaddles can be demonstrated convincingly by the following test. If a patient who has had a stroke is given a few quaddles on the extensor side of the finger joints of his or her spastically contracted hand, the spasm is relaxed immediately and the fingers stretch during the injection. Fig. 3.52 Injection sites to the lower extremities. 9. Quaddles on the Thigh and Leg From acupuncture we have learned to use a number of injection points in thigh and leg. We quaddle the inside of the thigh about the middle of the dorsal edge of the sartorius, and the inside of the leg over the posterior tibial artery and the medial malleolus. This treatment has proved helpful for abdominal and pelvic disorders, especially if they are accompanied by circulatory disturbances and signs of congestion in the legs; also for disorders affecting the hip and knee joints. In the thigh, we pass through the quaddles to give → (T) intramuscular infiltrations at a depth of 40–80 mm. In the leg, we try to reach as far as and into the posterior → (T) tibial artery (see Fig. 3.52). 10. Quaddles in Diseased Skin. For skin disorders such as localized eczema, psoriasis, shingles, local pruritus, scleroderma, etc., we set quaddles in the affected areas of skin. In these cases, subcutaneous infiltration is also indicated. Rheumatic conditions can often be cured permanently by one or several quaddles over the painful area, although no pharmacological explanation can be found for this effect. The same also applies to itching, weeping eczemas, etc. In cases of insect stings and snakebite, the sting or bite should be thoroughly infiltrated around and under the site of the lesion as soon as possible, in order to prevent toxic reactions. Retrobulbar injection See → (T) ciliary ganglion (p. 367). Sacral anesthesia See: → (T) epidural anesthesia (p. 298). Alternative terminology Trans-sacral infiltration, sacralplexus block. Anatomy See epidural anesthesia. Indications Sciatica, sciatica-like pain in carcinoma of the prostate and anorectal metastases, prostate and rectal disturbances, unilateral backache, circulatory dysfunctions of the lower extremities; sphincter spasm of the bladder, coccygodynia. Materials 60 mm needle. Quantity 2–5 mL. Technique The patient may either stand or lie face down. The line connecting the two iliac crests intersects the spinous process of the fourth lumbar vertebra. Another two spinous processes further caudally, we find that of the first sacral vertebra. The foramen lies two fingers’ breadths laterally from its lower edge. The needle is guided about 10 mm deep into the foramen. Following a negative aspiration test (liquor!), the procaine is infiltrated. The injection into the other foramina is given analogously. This conduction anesthesia of the sacral nerves through the second and third foramina blocks most of the area supplied by these in the sacral and coccygeal plexuses. (See Fig. 3.53.) Fig. 3.53 Injection to the first posterior sacral foramen. Indications Headache, vertigo, insomnia, post-concussion syndrome, traumatic epilepsy, cerebral arteriosclerosis, central vasospastic disturbances, diabetes insipidus, paralysis agitans (Parkinson disease), preand post-apoplectic states. Materials About a size 20 needle. Quantity 0.5 ml to each side. Technique For the injections to temples and parietal bone (10 mm superior to the center of the zygomatic arch [TB-22 = GB-3] or four fingers’ breadth superior to the center of the zygomatic arch, but one fingers’ breadth dorsally [ST-1]), one may first set → (T) quaddles bilaterally and then pass the needle through these, or simply insert the needle briskly and let it go down as far as (or into) the periosteum. The more briskly this is done, the less painful it is. If the patient reports other particularly painful points or areas on the skull, then these are proper sites for injection. No blueprint is possible and one should strictly avoid proceeding by rote. It is advisable to palpate the scalp thoroughly before injection and give special attention to any pressure-sensitive points. Amongst these the exit points of the → (T) supraorbital nerve above the eye and of the occipital nerve at the back of the head recur particularly frequently, and are especially important for our treatment. In acupuncture, for regulating the blood supply to the skull, the needles are set a fingers’ breadth above the center of the cheekbone. From this point it is also possible to suppress menstruation, and injection here during the first 3 days of a period should be avoided or the patient informed beforehand of the effect to be expected. Reference should also be made here to the hyperalgetic points in the atlas. These are found just in front of and below the mastoid process. Practitioners of manipulative therapy inject about 1.0 mL of procaine to the atlantal processes if their treatment has not succeeded in relieving pain in the atlas. We always combine the injection under the scalp with an → (T) intravenous procaine injection. (See Figs. 3.54, 3.55, 3.56.) Fig. 3.54 Injection points on the head, lateral aspect. Fig. 3.55 Injection points on the head, anterior aspect. Fig. 3.56 Frequently used points on the back of the head, neck, and shoulders. Indications: 1. Segmental therapy: All scars in the segment must be injected at the same time! In addition, any symptoms involving scars (itching, rash, inflammation etc.), keloids, post-operative symptoms. 2. Interference-field search: Any scar, of whatever kind and size, no matter how old it is and whether it has healed primarily or secondarily, often proves to be acting as an interference field for chronic disorders at remote sites. In cases of bronchial asthma, eczema, and other diseases that can appear during the first months or first year of an infant’s life, the first scar of every human being, the umbilicus, may be an interference field. This is particularly the case, if the health history lists omphalitis or draining umbilicus for the mother or slow healing of the umbilicus. In Chinese acupuncture, the view is held that a scar forms an obstacle to the vital force flowing through the body, and that this can be eliminated by the insertion of needles. 3. Prophylaxis: Anesthesia of surgical and injury scars, repeated twice or three times as soon as the wound has healed, is the best prophylaxis against their becoming painful and against the formation of keloids and interference fields. Materials Short needle. Quantity As required; for hard scars (especially on hands or feet), use a cartridge or locking syringe if possible. To keep the quantity of the neural-therapeutic product as small as possible, it is advisable to inject air into the scar beforehand. Technique The testing and injection of scars does not present any technical difficulties. Procaine is injected quite superficially into the scar so that something like a confluent weal (→ (T) quaddles) is formed. In the case of a long, narrow surgical scar, we set a line of quaddles about 10–20 mm apart. Sometimes the preparation will run along inside the scar of its own accord, separating the tissue layers along its length so that only very few separate injections will be needed. The two ends of any scar should always be located and treated with terminal → (T) quaddles. Extensive areas of scarring, e.g., resulting from burns, are preferably separated from the underlying tissue by first injecting air. This not only saves on the amount of procaine required, but also makes it easier afterwards to distribute the injected preparation more evenly over the area of the scar. This can be further helped by making circular massaging movements to distribute the procaine into the parchment-like crepitant skin emphysema. In the case of deeply indrawn scars, it is essential always to inject in depth. In the case of post-operative abdominal scars we go down as far as the peritoneum at one to three points. If the bone is also involved, the injection should include the periosteum; in nerve lesions and following neural surgery, we probe gingerly under continual infiltration for the nerve itself. Paresthesia will disappear following such treatment, and often the nerve function regenerates surprisingly well. There is, however, one exception to the rule: with deeply indrawn scars behind the ear after total mastoidectomy, avoid injecting directly into the crater or, if so, inject only very superficially! Because of the proximity of the meninges, too brusque an approach may produce vomiting and dizziness. In such cases it suffices to infiltrate around the scar in the surrounding area of healthy tissue and to inject to the adjacent periosteum. The term “scar” should be interpreted in the widest possible sense; so, for example, Dupuytren’s contracture is a scar. Every fracture heals with a bone scar, every extracted tooth, every enucleated eye leaves a scar that is capable of making the body sick. We must also search for any infiltrate left behind following the injection of tissue-irritant preparations. Even the scars left by corns, neck boils, toe surgery, or a perineal tear should not be left out of account. In newborns and infants, the umbilical scar can create an interference field, including conditions such as umbilical spasms, colics, dyspepsia, diarrhea, pylorospasm, enuresis, eczema, bronchospasm, chronic rhinitis, restlessness, etc. We place a large → quaddle above the umbilicus and infiltrate 2 mL 10–20 mm deeper and slightly lateral, between the layers of the rectus abdominus muscle. In adults, this can be done with 2–5 mL of 1% procaine or lidocaine solution, particularly, if a test injection into the umbilicus is described as extremely painful. Someone who is particularly thorough with regard to scars is certain to have a high success rate. Only if the whole of the scar is treated with the local anesthetic and air near the surface, and in a few places also at depth, and only if no part of it is left out, can a successful outcome be expected or the scar be safely eliminated as a relevant factor. It is even better to eliminate scars as potential candidates by injecting only the points found to produce a reaction, after measuring their electrical resistance or making an electrical skin test. Such reactive points generally lie at the two ends of surgical scars and especially on the edge of the scar. Kellner’s histological research indicates that anesthesia of a partially healed scar initiates a new granulocytic phase, which can lead to complete healing unless antigens or denatured body substances prevent the process. Talcum powder used on surgical gloves can form a foreign-body granuloma in a scar. These silicate crystals, and granulomas caused by suture thread, can occasionally produce such persistent interference that not even a series of neural-therapeutic injections will be adequate to desensitize the area completely. In such cases only scar excision can help. Although talcum is hardly ever used nowadays with surgical gloves, we should not forget that these crystals can still be present in old scars and can act as an interference field. See page 102 for a description of the electrical testing of scars. Anatomy The sciatic nerve receives its fibers from all the roots of the sacral plexus from L4 to S3. These roots combine in front of the greater sciatic foramen into a 35 mm-wide nerve plate. This lies extended on a bony base, so that it cannot move out of the way of our needle. This is of great importance to us, since we want to penetrate the nerve sheath and inject endoneurally. For the injection into the neighborhood of this nerve (perineural injection) we always need substantially greater quantities of procaine. But the sciatic nerve, with its tough connective tissue covering, places exceptional resistance in the way of any anesthetic solution given perineurally. Lidocaine contained in Xyloneural is about 10 times better able to penetrate the nerve sheath than procaine (Doenicke). Thus, if Xyloneural is used on any of the larger nerves, a perineural injection is adequate. In view of the small quantities we use, there is no cause to fear damage to the nerve as a result of administering a local anesthetic by direct intraneural injection. If there were, there would be no such thing as conduction anesthesia (nerve blocks). See Table 3.5 for a guide to diagnosing the level of the lesion in patients with intervertebral-disk sciatica and post-sciatic circulatory disturbances. The sciatic nerve provides the motor nerves to practically all the flexor muscles in the thigh and leg and to all the extensors in the leg and foot, and the sensory nerves to the skin of the leg and foot. Exception is a medial area from the knee to the medial edge of the foot, which is supplied by the → (T) femoral nerve. Indications Sciatica, damage to the intervertebral disks in the lumbar region; pain, circulatory disturbances and paresthesia in the lower extremities, paralysis following injection, and post-operative pressure paralysis. Neuralgia is always best attacked at the nerve root. This applies especially to the sciatic nerve, because it divides very high up. Neuralgia of the sciatic nerve starts in the region of the sacrum and initially often seems to be nothing more than lumbago. Later, the pain radiates to the leg. If the symptoms are exacerbated by straining, coughing, or sneezing, this suggests a prolapsed intervertebral disk. Bilateral sciatica always suggests a tumor in the true pelvis or disease of the lower vertebrae (metastases!). In the case of long-standing sciatica, when the pain in the region of the nerve root or the thigh is resolving, the patient often reports an unpleasant burning pain in the calf and finally under the lateral ankle bone. Our point of attack will therefore have to be guided by these circumstances. Technique In cases of sciatic disorders, the Huneke brothers injected to the sacral plexus only. This approach produced satisfying results. Later, Reischauer recommended injections more cranially to the sciatic nerve root. Also, see under → sciatica in Part II. The list below gives an outline of the sections to follow: 1. root of the sciatic nerve, p. 346; 2. → (T) epidural anesthesia, p. 348; 3. → (T) presacral infiltration, p. 348 (the advantage of this injection and of that to the lumbar → (T) sympathetic chain by comparison with injections to the sciatic nerve further down resides in the fact that the former also reach the pre-ganglionic fibers and ganglia); 4. sacral plexus, p. 348; 5. posterior → (T) sacral foramen and sacroiliac joint, p. 349; 6. gluteal region, p. 349; 7. branches of the sciatic nerve, p. 350. 1. Injection into the Region of the Root of the Sciatic Nerve L4/L5 and S1*** Materials 1 mm diameter 80 mm-long needle. Quantity Between 5–10 mL preferably from ampoules, because the preservative added in multiple dose vials may cause local irritation in paravertebral root anesthesia and cerebral irritation in inadvertent injections into the liquor (for example, root diverticuli). Level diagnosis and technique In the sciatic syndrome a major part is played by mechanical compression of the roots in the lumbosacral region, especially at L5 and S1. This must be treated first after identifying the level involved, until the acute stage has passed. The injections described under (4), (6) and (7) below are intended for treating the residual condition after the acute symptoms have disappeared, but they can also be used for purely neurogenic neuritis (lack of vitamin B, diabetes, toxemia etc.) and pseudoradicular syndromes (→ sciatica), but the latter are secondary with regard to the number of cases presenting for treatment. Before undertaking treatment, tumors, arthritis of the hip, and rheumatoid spondylitis must first be excluded. Apart from the patient’s history (interference field?), examination (typical posture), and palpation, the correct level for the injection is indicated above all by the spinous process that shows maximum sensitivity on percussion. But the band of pain indicated by the patient is also a useful pointer. The patient’s details should be checked against the guide for identifying the level of the lesion given in Table 3.5. The x-ray picture can provide some light on pathological processes, but should not be over-rated as a diagnostic tool. Before injection we orient ourselves by the line of spinous processes and the line of the iliac crest. These intersect over the spinous process of the fourth lumbar vertebra. The intervertebral disk L4/L5 and the root of L5 lie directly below this point. The injection must be given to the site of compression. This is where the prolapsed intervertebral disk is pressing on the root where it issues from the dura mater, but the exit point of the nerve from the vertebral foramen is one level higher! L4 Root Protrusion or prolapse of this disk is relatively rare. According to Reischauer, it occurs in only 5% of all sciaticas. The subjective band of pain runs from the anterior aspect of the thigh to the edge of the tibia. If the head of the patient with L4 syndrome is bent forward in a swift motion, while they are lying on their back, they will report lumbar pain caused by nerve stretch. The technique is similar to that used for L5, but the entry site for L4 is two finger breadths above the line of the iliac crest. (See Figs. 3.57, 3.58.) Fig. 3.57 Injection into the region of the root of the sciatic nerve; entry point to the canal between pelvis and fifth lumbar vertebra. Fig. 3.58 Injection into the region of the root of the sciatic nerve L3/L5 L5 Root The compression of this root causes 80% of all lumbar sciatica due to intervertebral disks. It produces pain on the lateral aspect of the thigh (“general’s stripe”) and leg down to the dorsum of the foot and the big toe. The patient complains of compression pain on coughing or sneezing, the Lasegue sign is positive. Maximum percussion sensitivity directly above the line of the iliac crest. Technique The 80 mm-long needle is inserted 10 mm above the line of the iliac crest and 40–50 mm laterally of the line of spinous processes. First proceed in a sagittal direction perpendicular to the skin surface and then converging 15 to the median plane, until the patient reacts with an electric pain in the L5 ligament when the needle reaches a depth of 50– 70 mm. After negative aspiration inject 5–10 mL at this point. If there is severe pain after 1–2 mL, it indicates that the needle has entered the extradural sheath of the nerve root. In that event, the needle should be withdrawn 3–4 mm. Alternatively, a 100 mm-long needle may be used to go down vertically below the line of spinous processes at the lumbosacral transition point. At a depth of about 50 mm, there is bone contact with the lateral process of L5. The needle should then be carefully passed round its upper edge and advanced in the same direction with 15–20 convergence relative to the median plane, until it reaches the posterior peripheral quadrant of the vertebra. While maintaining bone contact, 5–10 mL are injected there. If the needle encounters the cartilage of the intervertebral disk, the injection must not be given until bone contact is established above or below it. S1 Root Irritation of the S1 root accounts for 15% (36% according to W. Scheidt) of all lumbago and sciatica. The disk prolapse presses on the lower edge of the fifth lumbar vertebra where S1 exits from the dural sheath, whilst the nerve exit as such is the first sacral foramen. The band of pain extends from the posterior aspect of the thigh via the popliteal fossa, calf, heel, and lateral malleolus to toes three to five. Maximum sensitivity to percussion is found three fingers’ breadths caudally of the line joining the iliac crests. Pain is exacerbated by coughing and sneezing, Lasegue-Bragard is positive, the Achilles-tendon reflex negative, walking on tiptoe impossible. Technique The entry point is 40 mm laterally of the line of the spinous processes, two to three fingers’ breadths caudally of the line of the iliac crest between the lower edge of the lateral process of the fifth lumbar vertebra and the upper edge of the sacrum (Fig. 3.59). The 80 mm-long needle is advanced at an angle of 45° to the skin in a caudal direction and slightly convergent (20° to median plane) until bone contact is made. It is then at the intervertebral foramen or the lower edge of the fifth lumbar vertebra. Not later than on injection of 5–10 mL procaine or lidocaine solution, the patient indicates the typical pain in the posterior aspect of the thigh and radiating down from there. Fig. 3.59 Spinous processes that provide useful posterior landmarks. Posterior view. An accidental endodural injection into a saclike protrusion of the dura mater can generally be avoided by prior aspiration in two directions, turning the needle through 180 in between. The needle should never be at an angle exceeding 25 convergence to the median plane. But if, on a rare occasion, the dura is penetrated, this is not such a serious matter, since at the worst all that can happen from L3 down is lumbar anesthesia that paralyzes both legs while it lasts. The patient should be reassured and laid flat with their head raised. Their circulation should be closely watched and if necessary they should be given a peripheral vascular stimulant. Lumbar anesthesia as such is harmless and in such cases may be particularly effective. See possible complications on page 374. If the patient has been suffering from lumbago and/or sciatica for some considerable time or if after disk surgery a series of bilateral L5 and S1 anesthetics produces no positive response, this may be due to adhesion of a liquor protein precipitate in the lumbosacral peridural cavity (adhesive arachnitis). In such a case the solution injected can no longer diffuse as far as the longitudinal ligament. The adhesion can be dislodged by one or two → (T) epidural injections into the sacral hiatus of 30–40 mL 1% procaine solution. Once that is done, the injections to the nerve root can again become effective. Procaine treatment of the roots of the sciatic nerve raises the irritation threshold, which had become reduced by the mechanical irritation of the sympathetically innervated longitudinal ligament. The patient should be clearly informed that physical exercises and activity can help the healing process. Immobility is like poison for patients suffering from root sciatica! Let them move about and go for walks. In cases of long-term disorders, the iliosacral → (T) joint is always “blocked” and injections into this joint will help to reduce pain when moving. If the sciatic root disorder is followed by circulatory dysfunction in the lower extremities, we have to inject to the → (T) sympathetic chain at L3. Cauda Equina Compression Syndrome Collapse of the median mass can compress the cauda equina. The symptoms are severe pain, flaccid paralysis of both legs, sensory dysfunction (“saddleblock anesthesia”), paralysis of bladder and rectum, and absence of an Achilles-tendon reflex. Any attempt to use neural therapy in such cases is strictly out of the question. The patient must be referred as an emergency for immediate neurosurgery, since their fate may be decided in a matter of hours. In his Textbook of Neurology, W. Scheidt analyzes 559 lumbar disc prolapses with root involvement. His findings differ considerably from Reischauer. He lists the involvement as follows: L4: 5%; L5: 20%; L4 + L5: 7%; S1: 36%; L5 + S1: 18%; L4 + L5 + S1: 11%; cauda equina: 3%. 2. Epidural (Peridural) Anesthesia See page 330. 3. Presacral Infiltration See page 334. 4. Injection into and to the Sacral Plexus*** Anatomy The majority of the branches belonging to the sacral plexus, including the pudendal plexus, exit the pelvis through the infrapiriform part of the greater sciatic foramen: a. pudendal nerve, which leaves the gluteal area after 20 mm through the lesser sciatic foramen; b. sciatic nerve, which descends to the thigh, covered by the gluteus maximus muscle; c. posterior cutaneous femoral nerve, which follows the path of the sciatic nerve; d. inferior gluteal nerve, which runs inferior to the gluteus maximus nerve. Materials 1 mm diameter 100–120 mm-long needle. Quantity 5 mL. Technique To locate the sacral foramen, we need to draw two guide lines, the entry site for the needle being at their point of intersection: • The horizontal line runs from the top of the natal cleft to the upper border of the trochanter major. • The vertical line runs from the lateral dimple of the upper posterior iliac spine to the outer border of the ischial tuberosity. Fig. 3.60 Injection to and into the sacral plexus. The illustration shows the guide lines whose point of intersection marks the injection site: a: the horizontal line joins the upper border of the trochanter major with the top of the natal cleft; b: the vertical line runs from the upper posterior iliac spine (the lateral dimple of the erector spinae in the small of the back) to the outer edge of the ischial tuberosity. The dotted line shows the width of the nerve plate, the dashed line indicates the direction to the anterior gluteal tuberosity in which we find the various parts of the plexus. From the point of entry the needle penetrates vertically until bone contact is made. Caution: because in a rough approach, the tip of the needle can bend and the barb can tear nerve fibers. Since the nerve plate is 35 mm wide, we need to infiltrate from this point obliquely up and outward (10– 11-o’clock position left, 2–3-o’clock position right). There is absolutely no risk attached to doing so by advancing and withdrawing the probing point of the needle. The more thoroughly the 5 mL are distributed, the better the result will be. In proceeding as described, from below and medially upward in a lateral direction, the patient will note the following sequence of paresthesia, corresponding with the nerve plate of the sacral plexus (see Figs, 3.60, 3.61): • testicles, penis, perineum (pudendal nerve); then • thigh and buttocks; and finally • leg and foot. Since these injections affect muscle nerves, the patient needs to be supervised after the injection until the effects of the anesthesia have subsided and the patient is fit to participate in traffic. Patient and assistant have to be made aware of this. Fig. 3.61 Injection to the sacral plexus. Auxiliary lines to help in locating the plexus. 5. Posterior Sacral Foramen and Sacroiliac Joint See pages 341 and 309. 6. Gluteal Region** In the region of the buttocks the sciatic nerve lies exactly midway on the line joining the trochanter major and the tuberosity of the ischium. The patient lies on their sound side, with the sound leg extended and the affected leg flexed. The doctor now presses the left forefinger down on the site indicated and inserts the 100 mm-long needle, which must not be too thin, in front of the fingertip, guiding it in slightly obliquely upward until the patient reports a twitching sensation into the extremity. We give about 1 mL intraneurally and the same amount perineurally. Probing pressure of the thumb on the tuberosity of the ischium tells us whether there is a bursitis that is producing or accompanying the sciatic pain. If there is, we need to deposit a small amount of procaine where the probing point of the needle produces a particularly severe pain. 7. Branches of the Sciatic Nerve** Patients often complain about pain in the area of the calf, even after the sciatic disorder has been successfully treated. The sciatic nerve divides in the center of the thigh (or after its entrance into the popliteal fossa) into the tibial nerve and the common peroneal nerve. We can find the sciatic nerve before its division by palpating the popliteal artery at the level of the popliteal fold and inject one hand’s breadth above it. The nerve is located laterally to the femoral artery. We find the tibial nerve above the Achilles tendon in the lower third of the medial lower leg by firm pressure on the musculature there. We can also anesthetize it behind and below the medial malleolus immediately adjacent to the Achilles tendon laterally of the posterior tibial artery. The patient reports a twitching sensation forward as soon as we touch the nerve with the point of the needle. After creating paresthesias in the area that is supplied by the common peroneal nerve, we can locate and anesthetize this nerve directly below the head of the fibula. See peripheral → (T) nerves of the ankle and post-sciatic circulation disorder (lumbar → sympathetic chain). Sinuses, paranasal See page 213. Spinal anesthesia See: → (T) peridural anesthesia (p. 330). Spray treatment of nasal mucosa See: → (T) nasal spray (p. 314). Stellate-ganglion anesthesia See: stellate → (T) ganglion (p. 352). Supraorbital nerve See: → (T) nerves (nerve-exit points) (p. 315). The injections and complications listed below will be explored in the following section: 1. Sympathetic chain and its ganglia; a. injection to the stellate (cervicothoracic) ganglion, p. 352; b. injection to the upper and middle cervical ganglia, p. 359; c. injection to the lumbar sympathetic chain, p. 363; d. injection to the thoracic and sacral sympathetic chain, p. 364; e. possible mistakes and complications with injections to the sympathetic chain, p. 365; f. possible mistakes and complications with injections in the region of neck and chest, p. 365. 2. Parasympathetic ganglia of the head, p. 367: a. injection to the ciliary ganglion, p. 367; b. injection to the Gasserian (otic) ganglion and mandibular nerve, p. 368; c. injection to the pterygopalatine ganglion and the maxillary nerve, p. 369; d. injection to the submandibular ganglion, p. 371. 3. injection to the splanchnic nerves and the celiac ganglion, p. 371: a. possible mistakes and complications with injections in the abdominal and lumbar regions, p. 374. Injections into the sympathetic chain are a radical but also an extremely effective form of intervention to the neurovegetative system. Through this drug-induced temporary “sympathectomy,” we interrupt miscommunication and pathogenic impulse transmission. These injections act more rapidly, more effectively, and for longer than injections to the → (T) afferent arteries and nerves, and are considerably more extensive in their effectiveness (as, for example, also on the opposite side of the body). Anesthesia of the sympathetic chain blocks all the higher-order tonic impulses. In other words, the tonus level of the vessels is lowered and hence blood pressure is reduced. Vascular dilation improves the blood supply to the related peripheral region, and the pain-conducting autonomic fibers are temporarily blocked. Since these injections are a little more difficult and not entirely without risk, especially for the unpracticed, they should, as a rule, be considered only after therapy using all the other and simpler local measures has failed to produce the desired results! The question of whether injections to the sympathetic chain may also be given on an outpatient basis or whether they should be reserved only for in-patient treatment has been conclusively answered by the results achieved in the experience of the large numbers of neural therapists all over the world, in the sense that they can be used so frequently and so successfully to bring relief that they should be an essential part of every medical practitioner’s armory. Caution: If the patient is receiving long-term treatment with anticoagulants such as Marcumar, no injections to the sympathetic chain and its ganglia may be given whenever the prothrombin value is less than 45%. Special care is indicated where the prothrombin value is below 70%, when injections should be avoided in all but the most urgent indications, and vitamin K1 kept in readiness as an antidote. After the injection the patient should be kept under medical observation for several hours. Following anesthesia of the sympathetic chain, after blocking the action of vasoconstrictors, the blood pressure is reduced. Hence, in the case of hypotensive patients who may be liable to collapse, they should be kept under observation and, if necessary, kept lying down for a few minutes. However, in a German prisoner-of-war camp in Russia during World War II, Gerecht made a notable observation that would seem to contradict this. Of 300 practically moribund soldiers who were consigned to the camp suffering from starvation, which had practically reduced them to skeletons, and from shock due to cold injury, the first 100 died of circulatory failure despite medical treatment, since all cardiac and circulatory stimulants failed to act. When he noticed that, after two stellate ganglion anesthetics, a patient suffering from causalgia not only became pain-free but that his circulation, which had been in a state of total collapse, also made an astonishing recovery, he immediately gave all others who had arrived in the same desolate state a procaine injection to the cervical sympathetic chain, a paravertebral infiltration, or sacral anesthesia, depending on the most severe peripheral damage, and lost only one out of the remaining 200! One is tempted to conclude that in these cases sympathetic anesthesia produced a life-saving effect in what would normally be irreversible circulatory conditions and mobilized the last remaining reserves favoring survival. The best prophylaxis against possible complications is the use of small quantities for these injections and a perfected technique in their administration. This technique can be learned without undue difficulty. For a doctor to refrain from these injections out of consideration for his or her own safety would be so unethical that there cannot be any discussion as to whether he or she should proceed. Schmitt has said that with the right technique injections to the stellate ganglion, which for so little reason the general practitioner approaches with so much reluctance, are “no more dangerous than intravenous injections.” This applies to all the injections in this series! In very many disorders it is immaterial whether the injection to the sympathetic chain is given in the cervical or the lumbar region. What matters most is to produce a direct thrust into the neurovegetative system via this “vital nerve,” in order to correct the disturbed equilibrium. Generally, unilateral injection will suffice, possibly alternately left and right. With the small quantity of an average of 2 mL of procaine normally used per injection (except to the stellate ganglion) there is little to fear from injecting both sides at the same time, provided this is indicated and the correct technique used. As a rule, treatment is initially repeated weekly, and subsequently at longer intervals (e.g., if the symptoms recur). These anesthetic injections are also appropriate following resection of the sympathetic chain, since the sympathetic connections re-form soon after such an operation. We intentionally avoid describing our injections as “block” anesthesia. This term comes to us from neurology. The healthy cell is hyperpolarized by being supplied with chemical energy from the local anesthetic and is temporarily unable to respond to stimuli. This is known as an “anode block.” But in neural therapy, the decisive factor does not reside in any “healing anesthesia.” What we have to do is to produce a positive charge to chronically altered tissue whose pathologically reduced membrane-resting potential has become a permanent state. Here, the injection has an immediate recharging effect and acts to stabilize the cell potential. In other words, the cell membrane is protected against too rapid and too extensive a renewed discharge. In restoring normal bioelectrical conditions we are also reestablishing normal physiological conditions and once again link up the cell with the body’s normal information exchange system. However, this neurovegetative and bioelectrically rehabilitative effect can be produced only if we succeed in bringing procaine into previously damaged depolarized tissue. This is why the correct injection site is so important. In neural therapy we block nothing. On the contrary, we remove the block that is there and that has hitherto prevented the body’s self-healing powers from becoming effective. We need to remember again that small quantities of anesthetic are perfectly adequate for this purpose, since the healing stimulus to restore this equilibrium can be produced by quantities well below those required for anesthesia. Anatomy The sympathetic system comprises the sympathetic chain and its ganglia, the nerves leading from it and their plexuses. The sympathetic chain consists of: • the cervical chain with three ganglia, with branches to the carotid and subclavian arteries and to the heart; • the thoracic chain with branches to the aorta, the subclavian artery, the lung, and the splanchnic nerves; • the lumbar chain and its four or five ganglia; • the sacral chain and its four ganglia. The treatment of sympathetic nerves and ganglia is possible without blocking any spinal nerves, at the stellate ganglion, at the upper kidney pole in the region of the splanchnic nerve and at the lumbar sympathetic chain. 1a. Injection to the Stellate (Cervicothoracic) Ganglion*** Anatomy The sympathetic chain runs ventrally left and right of the vertebral column, from the level of the first cervical vertebra to the coccyx. In the cervical region it has only two or three ganglia. In 70% of all cases the lowest of these is combined into a single entity with the first thoracic ganglion and together with this forms the stellate ganglion. This is 10–30 mm long and 0.3–l mm thick. The ganglia of the sympathetic chain are linked interactively section by section via the communicating rami to the spinal nerves issuing from the spinal cord. The stellate ganglion lies on the head of the first rib, about 10 mm laterally from the first vertebral joint and 25–30 mm from the median line of the body. Amongst all the sympathetic ganglia, this ganglion occupies a place of special importance, for it is the largest neural control center apart from the central nervous system. It has afferent and efferent branches leading to the sympathetic thyroid and parathyroid plexuses, the phrenic, recurrent and vagus nerves, vascular branches to the vertebral, subclavian, and interior mammary arteries, and an accelerant nerve to the cardiac plexus. The stellate ganglion provides the autonomic innervation of the whole upper quadrant of the body. In addition to a purely regional effect, anesthesia of this ganglion alters the tonic state of the entire neurovegetative regulating system. Following anesthesia to the stellate ganglion, the quantity of blood and flow rate increase by 20% and oxygen saturation rises by as much as 50%. If repeated a sufficient number of times, this injection restores the equilibrium between vasoconstriction and vasodilation. Heppner stated that the disappearance of a cerebral edema following such an anesthetic suggested normalization of the endothelial barrier. The far-reaching importance of this control center is shown by the extensive list of indications for this injection. The therapeutic potential of treatment with local anesthetics in internal medicine, neurology, pulmonology, ENT, ophthalmology, and orthopedics is far too little used in practice. The reason for this is doubtless that many physicians are afraid of the risks inherent in this injection. This fear is groundless, as I hope to show. Indications Of primary interest are all arterial (not merely vasospastic) and venous circulatory dysfunctions, lymphatic occlusion in the head, the cervical region, and the upper part of the body, with their many possible sequelae, including pain due to autonomic causes, and trophic or tonic tissue changes: 1. In the upper half of the body: Frostbite, burns, slow-healing fractures, angiospastic circulatory disturbances of the Raynaud type, venous dysfunctions, lymphatic occlusion, puerperal mastitis, and hyperhidrosis; all pain symptoms in the head, chest, and arms, herpes zoster; to improve circulation after surgery. 2. Head: Pre- and post-apoplectic syndrome, cerebral edema and embolism, intracranial vascular spasms, certain forms of headache and migraine, post-concussional syndrome, dizziness, traumatic epilepsy, paralysis of the facial nerve, persistent facial edema following erysipelas; as supportive therapy in meningitis; venous thrombosis of the sinuses. 3. Eyes: Occlusion of the central retinal artery, thrombus of the central vein, diseases of the vascular membrane, abnormal pigmentation of the retina, degenerative disorders of the macula, ophthalmic herpes zoster, glaucoma etc. 4. Ears: Allergic disorders, frostbite, chronic suppurative otitis media, pressure in the ear, Ménière disease, deafness of the inner ear, tinnitus, sudden onset of deafness, otic zoster etc. 5. Nose: Vasomotor rhinitis, ozena, chronic suppuration of the sinuses etc. 6. Throat and neck: Hyperthyroidism, neuralgia, goiter, osteochondrosis of the cervical spine, cervical syndrome and all symptoms resulting from irritation of the cervical sympathetic system and of the nerve roots, rheumatic torticollis, septic tonsillitis, tonsillar abscess, tubercular laryngitis; to relieve pain in inoperable carcinoma of the larynx and hypopharynx. 7. Shoulder: Shoulder-arm syndrome, scalene syndrome, capsular arthritis, arthrosis deformans, subacromial bursitis, cervical migraine, post-traumatic stiffening of the joints, etc. 8. Arm: Brachialgia, causalgia, phantom-limb pains, brachial-plexus neuralgia, post-traumatic osteochondrosis; periostosis such as epicondylitis, styloiditis, tendinosis, painful joint dysfunctions, thrombosis and embolism in the arm, lymphatic edema following mastectomy, slow-healing wounds of any kind, acrocyanosis, Raynaud disease, obliterating thrombo-angiitis, joint pains, post-phlebitic edema and other circulatory disturbances; conditions following arterial injury. Also for diagnostic-prognostic testing before vascular surgery. 9. Lungs: Bronchial asthma, pulmonary hemorrhage, perforating injury to the lung, pulmonary edema (also in malignant hypertension with cardiac insufficiency in nephrosis and pre-uremia), tuberculosis of the lung, pneumonia, pleurisy, herpes zoster etc. In pulmonary embolism, procaine is injected bilaterally, with an interval of about 25 minutes between the two injections. 11. General disorders: Eclampsia (in addition to injecting to the stellate ganglion, an injection should also be given to the abdominal → (T) sympathetic chain at the upper renal pole), epilepsy, status epilepticus, hyperhidrosis in the upper part of the body, and other autonomic disorders. Possible contraindications Warning: caution is required in the case of bronchial asthma approaching decompensation. Fatal incidents have been reported! Anticoagulant therapy is an absolute bar against injections into the vicinity of any vessels. If possible, in recurrent nerve paresis, pneumothorax, or contralateral lung resection, the unpracticed should refrain from giving this injection, on the grounds of safety. In pulmonary emphysema and apical cavities, the only method that should be used is the one I recommend. Statistics The surgical unit of the Leipzig university hospital has confirmed the efficacy of stellate-ganglion anesthesia for disorders of the upper extremities. Two hundred and fourteen patients suffering from post-traumatic osteoporosis, cervical-spine syndrome, disturbed peripheral blood supply, epicondylitis, and humeroscapular periarthritis were treated eight times on average. An improvement was achieved in 76% of these, of whom 44% became completely symptom-free and in the other 32% all pathological changes regressed. There can be little doubt that any neural therapist with a perfect command of the method would have been successful in at least some of the 24% failures, by combining this with other injections in the segment or by the elimination of the interference field responsible. Materials For Herget’s or Leriche’s methods, 0.8–1 mm × 60 mm needle; Dosch: 0.7 mm × 32 mm (size 12) or 0.9 mm × 40 mm (size 1); Reischauer: 1 mm × 100 mm. Quantity 2–5 mL of a local anesthetic suitable for use in neural therapy is all that is required! The amount of 20 mL of 1% procaine solution used by surgeons is too large and can produce dangerous reactions simply by causing pressure on the nerves. Technique The relevant literature describes a total of 34 methods using the anterior, lateral, and posterior approaches! We shall limit ourselves to considering three of these that have proved themselves over the years: Herget’s Method* This is the preferred method in Europe. Because it is the best-known technique (see Figs. 3.62, 3.63, 3.64) in this part of the world, this will be described first. The patient lies on a flat support. They are instructed not to talk or swallow during the injection and not to move their head. A firm pad is pushed under their shoulders so that the head is bent straight back and the cervical spine is hyperextended. The entry point for the 60 mm-long needle is midway between the first ring of the trachea and the upper edge of the sternum, but two to three fingers’ breadths in a lateral direction on the inner edge of the sternocleidomastoid muscle. This site can also be found by dividing the distance from the mastoid process to the sternal attachment of the sternocleidomastoid into three equal parts. The entry point is then at the transition from the caudal to the middle third. When we have found this point, we let the patient breathe out and hold their breath, to let the lung move as far down as possible. The needle is inserted briskly in the direction of the projecting spinous process of the seventh cervical vertebra (vertebra prominens), which should be marked with one of the fingers of the free hand to ensure that the correct direction of thrust is maintained. At a depth of 40–50 mm we find a bony resistance, the head of the first rib. The point of the needle is now on the head of the first rib (or the lateral process of the seventh cervical vertebra), and the stellate ganglion lies in front of this. Fig. 3.62 Diagram showing the position of the stellate ganglion in front of the head of the first rib. Fig. 3.63 Finding the entry point for the injection to the stellate ganglion according to Herget. a. Divide the length of the sternocleidomastoid muscle into three equal parts. The entry point lies on the anterior edge of the muscle at the transition from the caudal to the middle third. b. At the level of the mid-point between the first ring of the trachea and the upper edge of the sternum, thence laterally to the anterior edge of the sternocleidomastoid muscle. The needle is withdrawn only about 1 mm, since an injection under the periosteum can result in considerable post-traumatic symptoms. If the needle enters the ganglion itself, the patient feels a dragging pain in the shoulder region. In that case, the needle should also be withdrawn 1 mm. Surgeons inject 10–25 mL, in order to “bathe” the ganglion and to include the middle cervical and the four uppermost thoracic segments of the sympathetic chain in the block. We know that such a large quantity can produce a dangerous carotid-sinus reflex when the neck is hyperextended back in this way, and believe that this is responsible for some of the accidents that have been reported. We inject only 2–5 mL of 1–2% procaine or of a 0.5–1% lidocaine solution such as Xyloneural. One reason is to avoid any risk of complications due to too large an amount of fluid; another is that the intended neural-therapeutic effect can be achieved equally well with these small quantities of low-concentration solutions (without the addition of vasoconstrictors!), amounts well below the threshold level for anesthesia. If the patient has a goiter, this can be pushed to one side, but no harm is done if the needle passes through the thyroid. Fig. 3.64 Injection to the stellate ganglion according to Herget. The division into three equal parts of the length of the sternocleidomastoid muscle and the clavicle is marked. The dotted line shows the apex of the pleura. If we want to block the cervical sympathetic chain directly, the needle should be lowered slightly in a caudal direction. But this is riskier and offers no advantage compared with that to the stellate ganglion, and is thus not recommended! We are already familiar with the most important safeguards. Caution: always aspirate before any injection in a cranial direction from the heart! Injection (particularly of larger quantities) into any vessel leading to the brain or into the liquor cavity can lead to dangerous complications! In order to avoid such complications and run no risk of accidentally injecting intra-arterially, we aspirate, turn the needle through 180 and aspirate again. It could happen that the needle is tangential and so close to the carotid or vertebral artery that the inner arterial wall is aspirated and closes the opening. In that event the patient could still receive an intravasal injection despite a negative result from aspiration. An added safeguard against the accidental administration of a relatively large quantity intra-arterially is therefore to inject only a few tenths of a milliliter of the local anesthetic while closely observing the patient for a few seconds. If they show no reaction, which might suggest an accidental intravasal injection or intolerance (nausea, vertigo, somnolence, tinnitus, or flashing in front of their eyes), the remainder can be injected briskly. Anesthesia of the stellate ganglion produces a homo-lateral Horner syndrome with ptosis, myosis, and enophthalmus: for anything from a few minutes up to 30 minutes, the pupil and the palpebral fissure will constrict, the eyeball sinks back, the homolateral conjunctiva reddens, and the secretion of sweat and tears ceases. There is active hyperemia of the vessels of the brain, face, and arm. Any joint pain that may have been present in the arm disappears. The eardrum is notably reddened. Given the small amount of procaine used, the appearance of Horner syndrome is a desirable reaction and is generally present. But it is not essential for the therapeutic effect, which need not run parallel with the extent to which a complete Horner syndrome is produced. After the injection, the patient should be kept under medical observation until they are once again fully fit for the road. It is part of the physician’s duties to draw their attention to this fact. When procaine is used, this will take about 30 minutes, in the case of lidocaine (because of its delayed resorption) this will increase to about 2–3 hours. With Carbostesin, 6–12 hours will be needed. When procaine is used, the Horner syndrome appears and disappears quicker. With lidocaine, the onset is slower but it lasts longer. This has no impact on the neural therapeutic effects. In patients who suffered apoplexia a long time ago, and patients with other nerve damage, the Horner syndrome does not take place with the initial infiltration, but during the course of the treatment it increases in appearance. It seems as if buried pathways are excavated and revived. For most therapeutic indications, a series of eight to 12 injections will be necessary, rarely more. The contralateral side should occasionally also be anesthetized. Normally, the injection is given only unilaterally. If it is intended to treat both sides in one session—required, for example, with pulmonary embolisms—we allow an interval of 30 minutes before the second injection when a 1% procaine solution is used. The Technique According to P. Dosch*** For several years, I applied the Herget technique as demonstrated to me by a surgeon using 10 mL Impletol. I did this until two incidents occurred in one day. I aspirated in one direction only and must have accidentally injected into the common carotid artery. I terminated the injection immediately when the patients fell unconscious for a few, never-ending minutes. One of them went into brief spasms. They finally came to with a retrograde amnesia but neither situation led to negative consequences. I was horrified and searched for a way to avoid pushing the needle blindly for several centimeters into the neck, which is rich with vascular and neural tissue, until contact with the bone is made. In a text by Leriche, I discovered a technique with an antero-lateral approach. In the original technique using an antero-lateral approach, which was developed jointly by Leriche, Fontaine, and de Seze, the patient lies flat on their back and turns their head only slightly to the opposite side. A felt pen or skin pencil is used to draw a line two finger breadths above and parallel to the clavicle and another along the lateral edge of the sternocleidomastoid muscle. The 60 mm-long needle is inserted at the point of intersection of these two lines and advanced almost vertically downward with only a slight deviation in a medial direction, until after 30–40 mm it makes bone contact with the head of the first rib or the lateral process of the seventh cervical vertebra. After a negative aspiration test, 10–20 mL of procaine (but we only give 2–5 mL!) are injected. I have adopted the lateral approach but have modified it considerably over time. The positioning of the patient’s head is different, the point of injection is approximately 2 cm higher and slightly more lateral, and the injection is not made directly to the ganglion but in front of the transverse process of the sixth cervical vertebra. By pressing down with the fingers, possible complications with the lungs, vessels, and liquor space are avoided. The injection fluid descends on the fascia of the throat down to the stellate ganglion. The onset of the Horner syndrome confirms this. This method is considerably simpler, safer, and can be used in daily outpatient practice. It worked for me for 35 years without complications. My sons and students apply this technique, which greatly expanded their therapeutic options. Technique In my method, the patient is seated on a chair with a neck support. If a neck support is not available, they simply lean their head against the wall after a pad has been placed under the back of their neck. They should lay their head back as far as they can and then turn it as far they can without difficulty in the opposite direction to the side to be treated. Now, the carotid tubercle should be easily palpable. On no account should the patient be allowed to turn the shoulder forward when they turn their head in this way or to draw in the neck out of fear! We now divide the sternocleidomastoid muscle from the mastoid attachment to the attachment to the sternum into three equal parts and mark the transition from the middle to the caudal third with a quaddle, on the outer edge of the sternocleidomastoid. Then, depending on the length of the patient’s neck, we place two or three fingertips of the left hand on the outer edge of the sternocleidomastoid in such a way that the caudal finger comes to lie on the upper edge of the sternoclavicular joint. The fingers are carefully pressed in to push the carotid artery and the jugular vein out of the way of the needle, and the distended apex of the pleura is also forced completely down and out of the way. This must be done gently and should not take too long, to avoid unnecessary irritation to the sensitive carotid sinus. Remember that a blow to this region in boxing can produce a knockout. (See Fig. 3.65.) Fig. 3.65 Diagram showing the position of the needle in carrying out the injection to the stellate ganglion according to Dosch. The finger in the cranial position on the neck can now feel the carotid tubercle of the sixth cervical vertebra as a small bony prominence and the patient pulls a face, because the pressure on the neurovascular bundle (formed by the common carotid artery, jugular vein, and the vagus nerve) against the bony substrate is unpleasant. Difficulty in locating the correct injection site can arise only if the patient has a very short, thick neck. In such a case, it is best to palpate the head of the rib by inclining the patient’s head to the side to be injected and thus relaxing the soft tissue. We leave the palpating finger in place and then incline the head back and to the opposite side into the prescribed position. If there is a goiter, this is pushed aside, but no harm is done if the needle goes through it. Only a 30–40 mm needle is used. This is inserted a short distance, immediately above the cranial finger, and is then guided deeper as far as bone contact, which is made practically subcutaneously! Even in the case of an adipose patient, if the needle fails to make bone contact at a maximum depth of about 20 mm, its position must be corrected without fail! The most important rule, by which we can avoid the only danger—that of accidentally injecting intradurally—is: throughout the injection with our short needle the palpating finger must maintain contact with the transverse process of the sixth cervical vertebra, and we must ensure that the point of the needle also remains in loose contact with it and does not slide further in! In view of the limited depth at which we need to work, a 20 mm-long needle would be adequate. But since this also has a diameter of only 0.4 mm, it provides no assurance with regard to the aspiration test. We therefore use a needle 30–40 mm in length and 0.7–0.9 mm thick, but this must not, of course, be inserted all the way. After bone contact, we withdraw the needle 1 mm, to avoid injecting subperiosteally, and aspirate in two directions by turning the needle through 180. After a negative aspiration result, in which no blood, air, or liquor must be aspirated, we inject a few tenths of a milliliter of the product and wait a few seconds while closely observing the patient. If they show no reaction that might indicate an intra-arterial injection or intolerance (nausea, vertigo, somnolence, tinnitus, flashing in front of the eyes), the rest of our 2–5 mL can be administered swiftly. Whenever the position of the needle is changed, or if the patient talks, swallows, or moves, we must aspirate again. In the seated patient, the local anesthetic descends along the prevertebral fascia to the stellate ganglion (cervicothoracicum). If, as the needle is inserted, the patient feels an electric shock radiating to their fingertips, then the needle is too far in a lateral direction amongst the strands of the brachial plexus. After correcting the needle’s position and observing the precautions stated, we may proceed with the injection. We sometimes seek out the brachial plexus intentionally for therapeutic reasons, but in such cases it is better to use a different method that includes all parts of this plexus (→ (T) nerves, afferent). If, on occasion, blood or liquor is aspirated, something that has never happened to me in 35 years, the needle must be removed at once and the injection should not be attempted again that day. With a little practice and used correctly, this beneficial injection is no riskier than an intravenous injection (Schmitt). After the injection of procaine, the patient remains sitting in the waiting room another half hour before being seen once more by the doctor and then being allowed to go home without hesitation. The advantages of the Leriche (Dosch) method (see Figs. 3.66, 3.67) compared with Herget’s are obvious: a. The patient is seated. Positioning them correctly calls for no special arrangements. With the patient seated, the apex of the lung lies further down than when they are lying down. If, in addition, we tell them to breathe out, it will lie even further down. Hyperextending the neck is unpleasant and can easily create a sensation of anxiety. Against this, the seated patient feels the injection to be considerably less unpleasant. b. When the patient turns their head, the common carotid artery and the jugular vein move medially out of the path of the needle, and when the fingers are pressed in, artery and vein are in any case both pushed completely out of the way (Fig. 3.66). Generally, the carotid tubercle (the well-developed anterior tubercle of the transverse process of the sixth cervical vertebra) can then be clearly felt further in, so that one does not have to penetrate into the unknown! The techniques of Herget and Leriche are directed more caudally for the head of the first rib in front of the body of the seventh cervical vertebra. My technique aims one vertebral level cranially for the transverse process of the sixth cervical vertebra. This provides greater safety through distance to the pleura, avoiding the risk to cause pneumothorax, which was a relatively frequent complication with the direction of the head of the first rib (Herget). Anatomical research has shown that the stellate ganglion is closely connected with the top of the pleura and even covered up to 70% by pleura (Hahn-Godeffroy, Jelisarowski). The only vital risk we can see is that of an accidental intradural injection, but this can be avoided not only by aspiration (hence never work with too thin a needle!) but also by observing the most important safety rule of all: when injecting, always maintain loose contact with the transverse process of the sixth cervical vertebra and take care not to slide further dorsally with the point of the needle. Neither of these is difficult to check and will prevent complications. c. In asthmatics and patients with emphysema, there is no risk of perforating the distended apex of the lung, since this is also pushed down and out of the way with the fingers. d. No special needle is required and it is even possible to administer this injection during a domiciliary visit to a bed-ridden patient. e. Provided a few simple precautions are observed, complications are practically impossible, and it is true to say that this injection is no more risky than an intravenous one. It has proved its worth in my own personal experience in over 50 000 injections, with not a single mishap. Because of its enormous action radius, every practitioner should be thoroughly familiar with it and use it daily, wherever it is necessary and indicated. Werthmann, a pediatrician from Salzburg, showed that my technique is successfully used with infants and toddlers. Parents and children usually reject lengthy segmental therapy, thus, the pediatrician has to search for quick results through few injections. The sympathetic chain can provide this option. Werthmann recommends my technique as safe and effective: “It can be performed under any condition.” Fig. 3.66 Diagram showing the injection site for the injection to the stellate ganglion according to Leriche’s method as modified by Dosch. The head of the seated patient is turned in the opposite direction to that of the injection and bent back. Two or three fingers of the left hand press in above the sternum on the outer edge of the sternocleidomastoid. The head of the first rib now lies almost subcutaneously above the topmost finger. In boxing, a blow to this area can produce a carotid-sinus reflex and may result in a knockout. Fig. 3.67 Injection to the stellate ganglion according to Dosch. From 1978 to 1982, Werthmann performed 2681 stellate injections and doubled that number in the following 4 years. In 8000 stellate anesthesias in children he did not experience one patient with an adverse reaction to procaine or any other type of complication. In some patients blood was aspirated; consequently, the injection was not performed on the same day. As particularly indicated for this type of injection, the experienced neural therapist lists the following: 1. Diseases of the respiratory tract are common in children. They are generally obstructive in nature. The Tiffeneau test immediately shows that the stellate infiltration improves air volume from 40–50–70% and more. This is objective proof of the positive results. In the case of mucoviscidosis (cystic fibrosis), the secondary symptoms can be improved. 2. In the case of sinusitis and sinubronchitis, secretolysis of the mucous membranes is also stimulated. 3. Until 18 months of age, developmental disorders with persistent foramen ovale and cardiac septal defects show improvement in 50–60% of the patients. Stellate injections support this improvement. Also, deeper breaths following the injection produce the hypobaric pressure necessary for spontaneous closure. The result is auscultatory and can be shown through phonocardiography (missing holosystolic murmur). 4. Spastic torticollis—in addition to local trauma of the head–neck area, the cause is damage to the extra-pyramidal system through encephalitis. 5. Reversible traumatic brain damage with motor restlessness or increased desire for sleep without desire to drink, following birth complications; in older children, post-concussional syndrome or conditions following meningitis and encephalitis. 6. Hearing disorders can be improved if they are detected and treated early. Every improvement is a success for the development of the child. This can be shown through children’s audiometry. Dittmar takes the view that perivasal infiltration around the → (T) subclavian artery with 2 mL of procaine is a complete substitute for the injection to the stellate ganglion. The post-ganglionic neurovascular fibers travel directly to the adventitial plexus of the subclavian artery. This way they reach the area supplied by the subclavia. By way of the → (T) vertebral artery this would include the area of the posterior cranial fossa and the inner ear. Although we hold this to be unlikely from theoretical considerations and, in the light of many years of experience, do not share his misgivings over the injection to the stellate ganglion, we are bound to point out this possible substitute. Reischauer’s Method* To avoid the possible risks of an accidental intradural injection, Reischauer found a dorsal approach. Technique The patient is seated on a chair, with the head inclined slightly forward. (See Figs. 3.68, 3.69.) The midline of the spinous processes is marked by a vertical line, then thin lines are drawn horizontally for the vertebra prominens (C7) and the spinous processes of C6 and T1. The 100 mm-long needle is inserted between C6 and C7 40 mm laterally of the line formed by the spinous processes, and is guided exactly at right angles to the surface of the skin and parallel to the median plane, to a depth of 40–50 mm until bone contact is felt. If it is intended to reach the root of C8, the point of entry is halfway between the transverse lines marking the spinous processes of the seventh cervical and first thoracic vertebrae. On making bone contact, the point of the needle is at the overlapping lateral segments of the cervical vertebral arches. It is then advanced carefully (under constant plunger pressure!) along these at an angle of 45 upward and laterally, i.e., in a divergent and cranial direction, until bone contact is lost, and can then be advanced (infiltrating) about another 10 mm in the fascial connective tissue of the tendinous muscle attachments, now, however, in a slightly convergent direction. It is then close to the root of C7 (or C8) and near the vertebral artery in front of the intervertebral foramen, but unable to enter it. The correct site for the injection is reached when the patient indicates the typical signal pain in the shoulder blade. Up to this point, about 2 mL will have been used, and another 2–3 mL are injected here. A Horner syndrome again indicates that the cervical part of the sympathetic chain has been successfully blocked. Reischauer himself used 20 mL of 1% procaine with this injection technique, to which he (unnecessarily) added Periston (collidon, PVP) to delay resorption. 1b. Injection to the Upper and Middle Cervical Ganglia** Anatomy The area of the neck is crowded with many, primarily autonomic afferent and efferent fibers. The upper cervical part of the sympathetic chain is located dorsally to the common and internal carotid artery, in front of the transverse processes of the second to fourth cervical vertebrae, embedded in the deep fascia of the neck. The vagus nerve, located slightly lateral to it, runs in the sheath of the large vessels. It branches out at the larynx and the heart and is connected to the cranial nerves IX (glossopharyngeal nerve), X (vagus nerve), and XII (hypoglossal nerve). Together with the external and internal carotid artery and the jugular vein, they also travel to the head. The vascular branches of the internal carotid plexus accompany all the branches of the artery to the inside of the skull and provide autonomic supply to the brain and the eyes. The caroticotympanic nerves branch off this plexus and form the tympanic plexus together with branches from the glossopharyngeal nerve. From here the minor petrosal nerve originates, which travels to the Gasserian (otic) ganglion and to the parotid gland. Another branch, the deep petrosal nerve, ends at the pterygopalatine ganglion. The external carotid nerves and their plexuses wrap around the artery of the same name, forming the external carotid plexus, which supplies the skull and its soft tissues, the larynx, and the thyroid. The visceral rami of the upper cervical ganglion are part of the cardiac plexus. Together with branches of the vagus and glossopharyngeal nerve, they form as pharyngeal rami the pharyngeal plexus, which supplies the pharynx, while the laryngeal rami enervate the larynx after connecting with the upper laryngeal nerve. Those areas of the head that receive their blood supply through the common carotid artery and its branches are affected by the upper cervical ganglion. Other areas that receive blood from the vertebral or basilar artery are innervated sympathetically by the stellate ganglion. Indications and materials Due to the anatomic circumstances, there is a long list of indications. This injection reaches the sympathetic and vagus systems, their ganglia and anastomoses, the glossopharyngeal nerve, the pericarotid plexus, the aortic and carotid depressor nerves, the carotid body, the hypoglossus and spinal nerves. Thus, the indications are equivalent to those listed for the stellate ganglion, the vagus, and glossopharyngeal nerve. It is used for cerebrosclerotic and vasospastic circulatory disorders in the entire area of the head, particularly the brain; also for central stress caused by a cerebral interference field due to encephalomyelopathias with headache, vertigo, and depression. In this way, it is a substitute for the → (T) cisternal procaine injection, which is actually illegal in Germany. It is also indicated in therapy-resistant complaints in the area of the cervical spine, migraine, and trigeminal neuralgias, particularly of the first branch; also for all allergic disorders (bronchial and cardiac asthma, eczema, hay fever, chronic urticaria). If simple injections fail, it is also recommended for pharynx, larynx, thyroid, ears, and eyes. It is indicated for interference fields in the head area, if they do not respond well to other measures because of secondary irritation of the head ganglia. Fig. 3.68 Reischauer’s method. The entry site in the method according to Reischauer lies between the spinous processes C6 and C7 40 mm laterally of the line of the spinous processes. Fig. 3.69 Anatomy and position of needle in the injection according to Reischauer. According to Descomps, anesthesia of this region affects the following: • Nervous reactions: These reduce the nociceptive centripetal stimuli, the centrifugal commands of autonomic reactions and badly adapted stimuli of a large number of regulatory systems by about half; they “relieve” the reticular formation and the cerebral cortex of an excess of subtle stimuli and prevent some hypothalamic reactions. • Hormonal, particularly pituitary reactions: It is surprising and at the same time exciting to find that disorders that retrostyloid anesthesia influences favorably and that are regarded as adaptation disorders respond to this quasi-physiological reactivation of what Selye calls the pituitary-cortex-suprarenal axis and that this is achieved without exogenous corticotherapy. The far-reaching effect of this injection becomes obvious when one considers that the upper cervical ganglion also supplies the pituitary and pineal glands (hypophysis and epiphysis) through the periarterial sympathetic pathways of the internal carotid plexus. Pituitary and hypothalamus form a morphological and functional control-circuit, i.e., centers in the hypothalamus are reached that are superior to the autonomic nervous system. They control, for example, temperature, blood pressure, and respiration, genital functions, wake–sleep cycle, lipo and hydrogen metabolism, and perspiration. The hypophysis produces approximately 20 different hormones and monitors and coordinates the finely tuned collaboration of all peripheral hormone glands. The endocrine pineal gland secrets noradrenalin, serotonin, and melatonin, which is exclusively produced there. Its secretion depends on the circadian rhythm: light reduces the melatonin and adrenalin production and increases the serotonin effect, which regulates the stimulation of the smooth muscles. Melatonin reduces the activity of the thyroid and stimulates the parathyroids. According to Pelz, anesthesia of the upper cervical ganglion has the following effects through the epiphysis alone: 1. Anovulatory cycle disorders, such as primary or secondary amenorrhea are eliminated. 2. Retardation of growth is removed, as long as the epiphysial plates are not closed. 3. The calcium level is increased through the parathyroid glands. 4. Thyroid hypertrophy, including Basedow, is normalized, if it is caused by pituitary or hypothalamus disorders. 5. Sclerodermia and Dupuytren’s contracture improve due to positive change in trophicity. 6. Some types of anemias and leukemias improve due the effect on the blood-producing systems. 7. Cerebral circulatory disorders improve; also post-traumatic epilepsy, optic neuritis following alcohol and nicotine abuse, etc. Contraindications In patients with dangerously high blood pressure with the risk of a stroke, an injection to the upper ganglion should be avoided. If the x-ray shows the sella turcica to be substantially constricted, severe headache must be expected to follow the injection, since the anesthetic produces vascular dilation in the pituitary region. Procaine allergy and the use of long-term anticoagulants (Marcumar, Sintrom, etc.) or other coagulation disorders prohibit this injection, so do diseases of the central nervous system, such as cerebral atrophy, brain damage with loss of tissue, multiple sclerosis, amyotrophic lateral sclerosis, and pyramidal signs (positive Babinsky reflex). Materials Needle: 60–80 mm length, 0.8 mm thickness. Quantity 2 (–5) mL. Technique a. In 1984, the neural therapist, J. Goebel, described a simple and safe technique for anesthesia of the upper cervical ganglion. He reflected on the fact that the posterior pharyngeal wall is separated from the deep neck fascia only by the narrow connective tissue of the retropharyngeal spatium. This allows one to maintain visual control over the needle, while closely approaching the ganglion through the mouth. The ganglion is located in front of the transverse processes of the second and third cervical vertebrae (Fig. 3.70). Before beginning the infiltration, the patient needs to be informed about the possible side-effects of the injection. This allows patients to prepare themselves mentally and avoid anxiety. The patient sits in a chair with a headrest against which they lean their head firmly. They need to open their mouth wide for good visibility. Fig. 3.70 Injection to the upper cervical ganglion (according to Goebel). Anatomical drawing: the ganglion is located in front of the transverse process of the second or third vertebra behind the internal carotid artery. Fig. 3.71 Injection to the upper cervical ganglion (according to Goebel). Drawing of the paratonsillar injection site in the posterior pharyngeal arch. Gagging and pain upon injection can be lessened and the mucous membranes disinfected through mucous membrane anesthesia with Gingicaine or Xylestesin spray. The patient needs to hold their breath while the spray is applied. After the spray has started acting, the 80 mm needle is inserted from the opposite corner of the mouth (approximately canine or first pre-molar of the lower jaw), i.e., from the left corner of the mouth when injecting the right side. Generally, the point of injection is located 5 mm medially to the (middle) edge of the tonsil or the tonsillectomy scar in the posterior arch of the palate (palato-pharyngeal arch) (Fig. 3.71). The direction of insertion follows the line from the corner of the mouth to the insertion point. After inserting the needle in this direction and advancing it 20 mm laterally and parallel to the horizontal line, its tip meets the body of the second cervical vertebra. The needle is retracted 1 mm. Now the first aspiration takes place and after the syringe is turned 180, the second aspiration takes place. This ensures that the tip of the needle is indeed placed extravasally and not pseudo-extravasally after aspiration of the intima. Now a test injection of only 0.2 mL is performed. If the patient does not report any sensation, 2 mL of a 1% procaine solution (or 0.5–1% lidocaine) are injected. If blood was aspirated, the needle needs to be removed immediately and nothing should be injected on that day. With the technique described above, an inadvertent injection through the intervertebral foramen into the dural space is not possible. The anesthesia should be performed only unilater-ally in 1 day. If both sides have to be injected, the first anesthesia has to subside (procaine 20–30 minutes, lidocaine 1–3 hours!) before the second one can be done. Fig. 3.72 Injection to the upper cervical ganglion. The injection site is located at the intersection of two lines: a vertical line downward from the anterior edge of the mastoid process and a horizontal line one fingers’ breadth cranially to the jaw line (technique according to Orsoni). Fig. 3.73 Injection to the upper cervical ganglion. a) Following Goebel’s technique, coming diagonally from the front, the needle travels perorally through the posterior pharyngeal wall, and ends behind the internal carotid artery. b) Following Orsoni’s technique, the needle travels first from the point of insertion (1) toward the opposite mastoid until it reaches the tip of the posterior tubercle of the third vertebral transverse process. From there (2) it needs to be advanced another 10 mm ventrally, in front of the transverse process. Injection after aspirations tests in two directions. Differing from Goebels’s technique (Figs. 3.70, 3.71, 3.73), R. Wander recommends moving the point of insertion medially to gain distance to the vagus nerve and avoid complications. The vertebral bodies of C2 and C3 are only 20 mm wide. Our target area is located only 10 mm lateral to the midline. Wander inserts the needle 10 mm lateral to the midline and slides along the circumference of the vertebral body until he reaches the transverse process. After negative aspiration, he injects into the arch between vertebral body and transverse process (personal information). b. If we follow Orsoni’s technique, we enter at the point of intersection of two auxiliary lines: a vertical line is drawn from the anterior edge of the mastoid process downward, the horizontal a fingers’ breadth above the mandibular angle. The 60–80 mm-long short-beveled needle is introduced at this point, at right angles to the surface of the skin, in the direction of the contra-lateral mastoid and, depending on the thickness of the soft tissue, will make bone contact at a depth of 30–40 mm. We are now at the tip of the posterior tubercle of the lateral process of the third cervical vertebra. The point of the needle is withdrawn slightly and advanced ventrally another 10 mm to a point in front of the lateral process. The technique is similar to that used for the injection to the stellate ganglion. The upper cervical ganglion lies close to the vertebra in front of the lateral process of the second and third cervical vertebrae, and the middle cervical ganglion is in front of the fourth cervical vertebra. Dura and spinal cord cannot be injured through the lateral injection because the intervertebral foramina are located anteriorly. The only risk is an inadvertent injection into the carotid and vertebral arteries that are located close by. This can be avoided through aspirating on two levels (with a 180 turn) and continuous aspiration control whenever the position of the point of the needle or of the patient (Fig. 3.2) is changed. Orsoni infiltrates this area with 10– 20 mL. In our view, a smaller quantity (2–5 mL) will achieve the same therapeutic effect whilst reducing the risk of a carotid-sinus reflex. c. Another technique from the side enters slightly further in a caudal direction: the patient turns their head to the side and stretches it back. The entry point is exactly halfway between the lateral edge of the greater horn of the hyoid bone and the lower edge of the mastoid at the level of the angle of the jaw. The needle is guided in a slightly medial-cranial direction as far as the anterior edge of the cervical vertebra. After bone contact it is withdrawn about 1 mm and the injection given after a negative aspiration test. Side-effects The patient needs to be prepared for side-effects that may occur while the most superior cervical ganglion is blocked through the effects of the anesthesia. Generally, a few minutes after the injection, Horner syndrome will be produced. If the injection was placed correctly and the Horner syndrome does not or only lightly occurs, severe regulation impairment can be concluded. This can be adjusted through additional injections. Increasing signs of Horner syndrome indicate improvement. When injecting procaine there will be a sensation of lightness in the head for about 20–30 minutes (when using lidocaine 1–3 hours). This is replaced by a sensation of warmth. Slightly impaired vision, heaviness of the eyelids, slight dizziness when walking, the sensation of a lump in the throat, and difficulty in swallowing, possibly even to the extent of a loss of voice (recurrent-nerve anesthesia) may be experienced. Occasionally a slight headache, lasting a day, is felt following treatment. The pulse is a little more rapid, the venous blood has been shown to become arterial in character, and the blood pressure temporarily increases by 20–30 mm (up to a maximum of +50 mm Hg). The blood calcium level also rises and sugar metabolism is increased. If treating a bilateral condition, up to 10 infiltrations may be necessary for a series of these injections, given alternately left and right three times a week. In stubborn conditions (e. g., with regulatory rigor following cortisone treatment) more series might be required. In addition, it is also advisable to look for a potential interference field. After the fourth or fifth treatment and for up to a fortnight after the final injection, the patient tends to feel sleepy and needs rather more sleep, but their fitness for work will not be impaired. This is to be regarded as a favorable sign and gives way to balanced relaxation, and may on occasion even reach a state of euphoria. Statistics In a group of 750 asthmatics and 81 other patients suffering from allergic disorders, Descomps achieved a 90% rate of cures or substantial improvements, which were maintained for at least 3 months, in 50% the success of the treatment was maintained for anything from 2–20 years. Twenty-five thousand of these injections were carried out without any lasting ill effects! d. We reach the middle cervical ganglion by entering at about the level of the annular cartilage, in order to arrive at the front of the lateral process of the fourth cervical vertebra. We push the sternocleidomastoid muscle and the vessels aside with the fingers of the left hand, aspirate and infiltrate as described above, but with only 2–5 mL of our neural-therapeutic preparation, when we are certain that we are outside any vessel. (See Fig. 3.74.) Fig. 3.74 Topography and position of needle in the injection directly to the sympathetic chain. 1c. Injection to the Lumbar Sympathetic Chain** Alternative terminology Lumbar “block,” lumbar sympathetic “block.” Anatomy Anesthesia of the lumbar sympathetic chain ganglia affects all sympathetic fibers supplying the lower extremities. The pre-ganglionic fibers originate at T12 through L2 and run through the anterior root of the spinal cord and white communicating branches to the sympathetic chain. From here, most post-ganglionic fibers pass as lumbar splanchnic nerves to the abdominal aortic plexus. Hence, the fibers of the leg travel in the plexus of the common and external iliac artery until they branch off around the femoral and popliteal arteries. As hypogastric plexus (presacral plexus) the fibers of the pelvic viscera continue the direction of the abdominal aortic plexus. Indications All chronic circulatory disturbances of the lower extremities, irrespective of their origin, form the classic domain for injections to the lumbar sympathetic chain, such as arterial occlusive disorders with possible gangrene formation, conditions resulting from burns, frostbite, varicose ulcers, ulcerating scars, ulcers due to x-rays or bedsores, causalgia, slow-healing amputation stumps, amputation-stump neuralgia, phantom-limb pains, hyperhidrosis, painful stiffness in the joints, post-traumatic osteoporosis, Sudeck dystrophy; in vascular spasm for opening a collateral circulation. Further indications are venous insufficiency with acute or chronic thrombophlebitis and post-phlebitic edema, embolism; in impotence, for improved blood supply to the genital organs. The injections are also worth trying in spinal motor paresis. When the technique is used correctly, the patient reports a sensation of warmth in the leg on the side of the injection, and this can also be felt objectively. In addition to effecting an improvement in the arterial blood supply, we also achieve an interruption of the sympathetic pain-transmission channels that is maintained well beyond the limited duration of the anesthetic effect. Fig. 3.75 Position of the sympathetic chain in the thoracic and lumbar area. Injection to L3 in Post-sciatic Circulatory Disturbances Reischauer found that 26% of patients who had suffered from L5 intervertebral-disk sciatica, one of those that occur most frequently, also had “post-sciatic circulatory disturbances.” These are characterized by sciatica-type pain in the sacrum and the outer and flexor sides of the thigh, and dysbasia (intermittent claudication) with poor circulation in the lower extremity. There are no compression symptoms (pain is exacerbated when the patient sneezes or coughs), the vertebral column is mobile, the spinous processes are not percussion-sensitive and arteriographic findings are negative. The patient’s history includes references to lumbago and sciatica. The only recommended treatment consists of repeated injections of procaine or lidocaine to the lumbar sympathetic chain at L3. The radiating pain reminiscent of sciatica, dysbasia, and the circulatory disturbances disappear immediately following the injection. The latter, of course, are not due to any organic damage to the vascular wall but a sympathetic irritation caused by compression of L5, which maintains spastic vasoconstriction in the capillary terminal reticulum. This can be eliminated only by this therapy. Treatment should initially be repeated at intervals of 1 or 2 days, and at ever-increasing intervals as the condition improves. If these injections fail to provide relief, several → (T) peridural anesthetics should be administered in hospital. These are even more effective. Materials 1 mm × 100–120 mm-long needle. Quantity 2–5 mL. Technique The standing patient places their hands on a table and bends over. The line of the iliac crest is drawn on their back, and at right angles to this the line of the spinous processes. The line connecting the two iliac crests marks the spinous process of the fourth lumbar vertebra. From this we can count off the required level. The needle is inserted three finger breadths laterally from the line of the spinous processes, or at L3 three finger breadths above the line of the iliac crest. In the lumbar region, the upper edge of the spinous process corresponds approximately to the lower edge of the lateral process. When the 100–120 mm-long needle is advanced at a convergent angle of about 25 to the median plane, it makes bone contact at a depth of about 30 mm and has reached the lateral process. In that event, the needle position has to be corrected to bypass this. The needle is then advanced by infiltrating under constant plunger pressure and reaches the lateral surface of the vertebral body at a depth of 80–90 mm (50 mm from the lateral process). The point of the needle is now moved carefully past this until contact is just lost with the convex surface of the vertebra. It is then immediately adjacent to the sympathetic chain. Following negative aspiration we set a depot here of 2–5 mL (see Figs. 3.74, 3.59). 1d. Injection to the Thoracic and Sacral Sympathetic Chain* Indications, technique, and materials See the section on injection to the lumbar sympathetic chain above. The Russian school (Vishnevski et al.) has shown us that the thrust into the system with a procaine or lidocaine injection directly to the sympathetic chain is perfectly capable of producing a reaction at even a very remote site. Thus, it is not of major importance to the therapeutic processes at what level and on which side we inject to the sympathetic chain! Incidentally, it is worth remembering that the thoracic sympathetic chain is located more on the lateral surface of the thoracic vertebrae, whilst the lumbar sympathetic chain lies further on the anterolateral surface. See Figure 3.75. Warning! We have to caution against injections to the thoracic sympathetic chain ganglia because they are prone to complications. In this area, an involuntary subdural injection causes life-threatening complications! The pelvic part of the sympathetic chain contains three to four sacral ganglia and, at the caudal end, the solitary ganglion impar. In this ganglion, both sympathetic chains come together in an arch-like shape. We inject to this ganglion when we treat → coccygodynia. The ganglion is located in front of the coccyx. To reach the ventral side of the coccyx, we need a bent needle. Before removing it from its packaging, we bend the 60 mm disposable cannula into a semi-circle. 1e. Possible Mistakes and Complications with Injections to the Sympathetic Chain Outdated statistics may create the impression that injections to the sympathetic chain and its ganglia are full of risk for both doctor and patient. Out of 10 000 stellate-ganglion anesthetics, Luzuy reported only a single fatality (in bronchial asthma with marginal decompensation). Leriche wrote: The risks of injections to the stellate ganglion have often been exaggerated; even sudden death has been reported. Personally, in several thousand stellate blocks, I have observed nothing of the kind. I attach a certain amount of importance to the psychological state of the patient. It is an important part of our duties to reassure him and not to awaken in him the impression of a difficult and dangerous act on our part. Further, one must be completely familiar with the technique. A survey made amongst neural therapists with an average of 20 years’ experience showed that in 1.75 million anesthetics to the sympathetic chain and its ganglia we had not a single case of irreversible damage as a result of our therapy, much less a fatality. These included about 500 000 (estimated) injections to the stellate ganglion. In these, there were 12 (1 in 41 667) accidental intra-arterial injections, which produced brief convulsions with loss of consciousness, all of which were without sequelae and required no further treatment. One colleague admitted that he had completely omitted to aspirate before the injection. The method can hardly be blamed for such gross negligence. On 20 occasions (1 in 25 000), there was a pneumothorax. These healed within a few days, some of them under the supervision of a specialist, the rest with only minor symptoms and without treatment. In about 500 000 injections to the upper renal pole, there were 25 cases of macroscopic hematuria and one renal colic. Three cases of severe after-bleeding were referred to hospital as a precaution, but all patients were able to be discharged 2 or 3 days later. Only on one occasion was there a massive retroperitoneal hemorrhage, which called for lengthy hospital treatment but also resulted in no permanent damage. This patient suffered from a previously unrecognized coagulation defect. Our figures are not statistics; they merely provide a general view. As far as I know, there has not been a single fatality attributable to the method in over 65 years of neural therapy. If the technique is used in accordance with the rules, and if the physician has an adequate mental picture of the topography and reasonably sensitive fingertips, complications can be easily avoided. Nevertheless, no matter how well things may have gone on so many occasions, these injections must never become a light-hearted routine matter. We must concentrate fully on what we are doing whenever we administer them to our patients and proceed in a thoroughly responsible manner. Only if we do this, will nothing happen. Every medical intervention harbors a certain element of risk, and it is essential to know what it is in order to reduce it to its absolute minimum. Caution: If the prothrombin value (Marcumar) is below 45% (–70%), do not give any injection to the sympathetic chain! 1f. Possible Mistakes and Complications with Injections in the Region of Neck and Chest • If a local anesthetic is injected into a vessel leading to the brain, a toxic convulsion effect can be produced, which may be fatal when substantial quantities are injected, although this would seem to be contradicted by recent reports on therapeutic procaine injections into the → (T) carotid artery. Despite this, it is best to avoid injections into any vessels that lead to the brain. Thus we must always aspirate, preferably twice and in opposite directions, before any injection and whenever we change the position of the needle or if the patient has moved (coughing, swallowing, etc.). No blood must be aspirated. It is preferable to check 10 times too often than miss once, and to inject only when one is certain that the needle is lying extravasally. We can be certain that it is correctly sited only if there is no reaction when we give the patient a preliminary injection of a few tenths of a milliliter. Particularly when there is an occlusion or considerable narrowing of the internal carotid artery, blood might reach one of these vessels indirectly. In rare cases, existing anastomoses can cause retrograde blood flow into the skull. There is a greater chance for this to happen if large amounts of fluid are injected quickly with high plunger pressure! In the area of the head and the face, the number of vascular anastomoses is rather large. Anastomosis can take place, for example, in the area of the nose, between the terminal branches of the internal carotid artery, such as the ophthalmic artery, and the terminal branches of the external carotid artery. In the circle of Willis, the internal carotid artery and the vertebral artery form anastomoses at the base of the midbrain. As a result: we never inject larger amounts bolus-like, but, particularly in the area of the head, slowly and cautiously. With a little experience it is easy enough to acquire the proper “feel.” • Incidents occurring with injections in the cervical region need not, however, necessarily be due to an accidental intravasal or intradural injection. The cervical region is a great deal more sensitive than any other part of the body. Especially in the hyperergic form of reaction encountered amongst autonomically severely disturbed patients, the carotid-sinus reflex can lead to unpleasant shock reactions with loss of consciousness and tonic-clonic twitching. Such incidents are exceedingly rare, but they can be fatal if the amount injected is excessive. Fortunately, they generally pass off without treatment. It has been stated that such a state of shock can be arrested by the intravenous administration of soluble corticosteroid preparations (e.g., Solu-Decortin H). Instead of 15–25 mL, we use only 2–5 mL without the addition of vasoconstrictors, which increase the toxicity of the local anesthetic by a factor of 10. By pressing in with the fingers (Dosch method) we avoid injury to vessels and the apex of the lung. • According to Koster and Kasman, the frequently voiced concerns about damage through ascending procaine to the medullary centers resulting from unintentional sub- or intradural injection are unfounded! Even during complete anesthesia of the upper extremities and the head, the amounts and concentrations required for spinal anesthesia never affect the respiratory center. The authors concluded that the respiratory center possesses properties that make it insensitive to procaine. However, one ascertains by aspiration before injection that the needle is not lying intradurally. Because paravertebral injections and injections to the sympathetic chain in the thoracic area are prone to complications, we prefer to replace them with anesthesia of the stellate ganglion. Occasionally, when the anatomical conditions are unfavorable, the dural sheath may extend as far as the intervertebral foramina. In these extremely rare cases, one need not penetrate into the foramina in order to place the needle intradurally. If this happens due to inattention or if an injection is made into the spinal cord, irreversible damage, even death, may occur. When injecting to the stellate ganglion, this can be prevented not only by aspiration in two directions but also by using a short needle and maintaining constant bone contact with the head of the first rib or the carotid tubercle of the sixth cervical vertebra. • In cases where hypotonic patients have been given procaine injections, a dangerously reduced blood pressure has been observed, which has been due to peripheral vasomotor paralysis and damage to the nerve centers. With procaine and the extremely small quantities we use, there is little risk of this. Larger quantities can produce a carotid-sinus reflex. Nor should the pressure exerted with the fingers to move aside the bundle of vessels and nerves be too brusque or maintained for too long. I have had personal experience of this reflex in three autonomically labile patients, including a perforation of the carotid artery (but without injecting into it): nausea, blackness, and a spinning sensation in front of the eyes, throbbing in the head, and buzzing in the ears, pallor, and dilated pupils. These symptoms passed off without further treatment after lying the patient flat for a few minutes. If there is a more severe reaction, none of which I have ever experienced personally, although I have given countless injections into this area, the intravenous administration of a cortisone preparation (e.g., Solu-Decortin H) would be indicated. • Setting a pneumothorax by perforating the apex of the pleura. This complication can be avoided by pressing the palpating finger into the carotid tubercle of the transverse process of the sixth cervical vertebra, injecting while maintaining loose bone contact (Dosch) and inserting the needle in full expiration. Often enough the patient does not even notice that he or she has a pneumothorax, and it will heal by itself with no further treatment, without affecting the patient’s general condition to any extent; at worst it may require 2 or 3 days in bed or perhaps reduced activity. A tension pneumothorax is hardly likely. Should it occur, it would present with symptoms of chest pains, an irritable cough, a sensation of constriction, shortness of breath, and blood in the sputum. In addition to the administration of sedatives, the patient should be referred to hospital for in-patient treatment (paracentesis, decompression). • Hoarseness or loss of voice following injections in the cervical region. The injection has been given too far medially, and the recurrent nerve has been anesthetized. This is harmless and passes off quickly. The same also applies to anesthesia of the phrenic nerve, which temporarily stops movement of the diaphragm. Care is therefore recommended if injections are given bilaterally. • If the injection is accidentally given into the vertebral artery (and this is easily avoided by prior aspiration!), the patient will temporarily have tinnitus and/or flashing in front of the eye on the side of the injection. This, too, passes off quite rapidly and does not call for treatment. 2a. Injection to the Ciliary Ganglion** Alternative terminology Retrobulbar infiltration, ciliary anesthesia. Anatomy The ciliary ganglion has a length of 2–3 mm. It is not always found in the same position in every patient. Its location is about 10–20 mm behind the eyeball (which has a diameter of about 24 mm), generally directly laterally of the optic nerve and medially of the origin of the lateral rectus muscle of the eye. Directly behind the ganglion, the ophthalmic artery comes from a lateral direction to turn around the optic nerve and continues above it. This ganglion receives sensory fibers from the nasociliary nerve, parasympathetic fibers from the oculomotor nerve, and sympathetic fibers from the upper cervical ganglion, via the internal carotid plexus and the ophthalmic plexus. The fibers of the ciliary ganglion run to the eyeball and supply the vessels of the choroid and sclera, and some of their branches run to the ciliary muscle, the sphincter, and dilator muscles of the pupil and to the iris and cornea. The ciliary body produces intraocular fluid, which nourishes large parts of the eye, including the external layers of the retina. The intraocular pressure depends on the balance between production and drainage of this fluid. This regulation is based on sensible information coming from ganglion cells that are located in the ciliary body. Indications 1. Segmental therapy: All acute inflammatory and chronic eye disorders, e.g., neuritis of the optic nerve, scleritis, keratitis, iritis, iridocyclitis, retinal periphlebitis with vitreous hemorrhage, rheumatic–allergic reactions and post-traumatic dysfunctions, glaucoma (except for hydrophthalmia), venous thrombosis of the retina. In addition, for cases of the following, where simpler injections (intravenous, quaddles, nerve-exit points) have failed to produce results: conjunctivitis, ophthalmic herpes zoster, all forms of corneal disorders, disorders of the retina (except detachment), incipient cataract, certain forms of headache and recalcitrant neuralgia in the region of the eye. Apart from eliminating the inflammation and reducing pressure on the eye by regulating the autonomic state, both of which are of great importance, we are also able to improve the blood supply to the optic nerve, retina, and the anterior parts of the eye. 2. Interference-field search: As test injection, if the patient’s history suggests the presence of an interference field in the region of the eye, e.g., due to eye injury, eye surgery, persistent or repeated inflammatory conditions. Materials There is no need for a specially curved needle, a straight one 35 mm to a maximum of 40 mm in length will do. If it is 40 mm long, it must not be inserted all the way! Ophthalmologists use a special straight 35 mm needle available commercially, with a slightly rounded tip. Quantity 1.5 mL procaine solution, e.g., 1% Novocaine for therapeutic use made by Hoechst Pharmaceuticals. The more recently developed local anesthetics based on amides are less suitable for this injection, at least for outpatient treatment in a general practice, because they have a longer-lasting anesthetic action. Fig. 3.76 Injection to the ciliary ganglion. Orientation on the bony skull. Fig. 3.77 Injection to the ciliary ganglion. Technique The seated patient has the back of their head supported by a neck support or by an assistant who holds it firmly in position, or they lean it against a wall. They hold their eyes open and look slightly upward and medially. This causes the inferior oblique muscle to be tensed so that the needle can be guided through below it. The physician carefully pushes the tip of his or her forefinger into the lower lid in such a way that the eyeball is pushed away upward and toward the nose. The needle is inserted in the outer lower corner of the eye socket through the skin of the lower lid, i.e., at about the 7-o’clock position for the right eye, and at about the 5-o’clock position for the left eye. (See Figs. 3.76, 3.77.) We best avoid injury to the eye by first inserting the needle and guiding it approximately parallel to the lower orbital wall and sagitally to a depth of about 20 mm. In order to push any vessels out of the way, we always maintain slight pressure on the plunger as we proceed. We then advance the needle another 10–15 mm whilst raising it slightly back up and inward toward the orbital foramen where the optic nerve enters. The point of the needle now lies in the retrobulbar space within Tenon’s capsule (fascia of the bulb of the eye), immediately in front of the ciliary ganglion. This will do; there is no need to pass into the ganglion itself. The distance from the entry point to the site of our injection is 35 mm. Thus, the 40 mm-long needle must not be inserted all the way, or there will be a risk of injury to the ophthalmic artery. During the injection, the patient should avoid moving his or her eyes, as this would increase the risk of injury to a vessel. After short aspiration, the remaining amount of about 0.7 mL of procaine solution is injected quickly. This is quite simple and not dangerous if the correct technique is used! The general practitioner will rapidly lose his or her first misgivings about this slightly unusual injection. All that can happen (apart from an exophthalmus, which, however, in view of the small quantity of local anesthetic used, will be brief) is a retrobulbar hematoma. According to Piotrovski, with the correct technique, a hematoma may occur on average once in 80 injections. This is almost always a relatively harmless venous hemorrhage, which shows within the first 5 minutes and stops by itself by autocompression. It then acts like an additional → (T) autohemotherapeutic treatment. It is, however, advisable to tell the patient casually beforehand that this might happen, i.e., that if he or she is unlucky he or she may get a black eye following this important injection. The visual disturbance that follows is caused firstly by dilation of the pupil and secondly by an outward squint (due to the temporary paralysis of the muscles of the eye). When procaine is used, this will disappear in 30 minutes without any negative sequel, whilst it will persist for 2 hours or more after Xylocaine. In the relevant literature, incidents have been described in which, following a retrobulbar injection (mostly when the needle has been inserted further than 35 mm!), arterial hemorrhages are said to have occurred, with the orbit completely filled, severe bulbar protrusion, and the risk of occlusion of the retinal vessels. Such cases would have to be treated by an eye specialist. According to Killian, by using the technique just described, these complications can largely be avoided. Cases of generally temporary blindness following local anesthesia with adrenalin additives (such as are customary in eye surgery) have also been reported (Doden). This is therefore the appropriate place to warn yet again against using anesthetics with vasoconstrictor additives in neural therapy! No case has come to my knowledge of permanent damage to the eye resulting from neural-therapeutic ciliary anesthesia. In some cases it can be useful to combine injections to the ciliary and the pterygopalatine ganglion because the latter supplies the eyeball and its auxiliary organs with autonomic fibers as well. Anyone who is concerned to provide relief to sufferers from disorders of the eye must learn to give this injection and to use it frequently! 2b. Injection to the Gasserian (Otic) Ganglion and Mandibular Nerve* Alternative terminology Injection to the oval foramen, injection to the root of the third branch of the trigeminal nerve, injection to the Gasserian (otic) ganglion. With this injection we do not in fact reach the Gasserian (otic) ganglion itself, since this lies intracranially. To be precise, we only inject into the mandibular nerve, the largest of the three branches, immediately after it leaves the oval foramen. Anatomy After leaving the skull through the oval foramen, the mandibular nerve divides into several branches. The strongest branch is the inferior alveolar nerve that supplies the teeth of the lower jaw and continues as the mental nerve to the lower lip and the chin. Another main branch is the auriculo-temporal nerve. The lingual and buccal nerves supply the mucous membranes. The motor branch supplies the mylohyoid muscle and the muscles used for mastication including temporalis, masseter, lateral, and medial pterygoid muscle. The branch that supplies the latter is located most medially and supplies the tensor tympani and veli palatini muscles. At this nerve branch lies the parasympathetic Gasserian (otic) ganglion. It supplies the parotid gland and the buccal glands and receives its pre-ganglionic fibers from the vagus nerve. We also anesthetize this ganglion whenever we inject to the mandibular nerve. Indications: → ear. Indications Trigeminal neuralgia, if injections to the → (T) nerve-exit points have failed to produce the desired results; trismus, pain due to malignancy in the area supplied by this nerve; also worth trying with headaches of uncertain origin. Materials 0.8 mm diameter × 60 mm needle. Quantity 1–2 mL. Technique The seated patient leans with the back of his or her head against the head rest and opens and closes his or her mouth several times, to enable the doctor to palpate the mandibular notch directly below the center of the zygomatic arch. The dimple formed as a result below the cheekbone and above the notch is the entry site for our injection. It is about 30 mm in front of the tragus. The patient should now keep the mouth half open. The needle is inserted a short distance and is then guided transversally (on the opposite side) along the base toward the middle of the base of the skull. At a depth of about 40 mm the needle meets the pterygoid process. The depth reached by the needle is noted; it is then withdrawn slightly and guided carefully about 5–10 mm further in a dorsal direction. At a depth of 50 mm we are now near the oval foramen (see Figs. 3.78, 3.79), and after prior aspiration the procaine is deposited here (beware of blood!). The patient’s pain reaction shows when the mandibular nerve has been reached. Paresthesia occurring in the region supplied by this nerve indicates that the needle has been sited correctly. Fig. 3.78 Injection to the oval foramen (Gasserian [otic] ganglion). The patient holds his or her mouth half open. The needle is inserted below the center of the zygomatic arch above the mandibular notch and is then advanced to the center of the base of the skull to a depth of 40–50 mm. Fig. 3.79 Injection to the oval foramen (Gasserian [otic] ganglion). Orientation on the bony skull. The point of the needle lies in front of the separation of the mandibular nerve into its two branches. 2c. Injection to the Pterygopalatine Ganglion and the Maxillary Nerve** Anatomy: • The parasympathetic pterygopalatine ganglion is located in the pterygopalatine fossa, directly below the maxillar nerve. The ganglion has three roots: the parasympathetic major petrosal nerve that originates in the facial nerve, the sympathetic petrosus profundus nerve that originates in the carotid plexus (which also provides a connection to the ciliary ganglion), and the sensory pterygopalatine nerves that originate in the maxillary nerve. Post-ganglionic fibers run with branches of the maxillary nerve to glands that are located in the mucous membrane of the palate, nasal cavity, and paranasal sinuses. After traveling along a complicated pathway, they reach the lacrimal gland. • The maxillary nerve carries only sensory trigeminus fibers. It supplies the dura mater, the skin of the lower eyelid, the cheek, the upper lip, and the outside of the nose, as well as the teeth and gum of the upper jaw. After branching off the trigeminal Gasserian (otic) ganglion, it enters the pterygopalatine fossa through the foramen rotundum. Here, it divides itself into its three main branches: pterygopalatine nerves, infraorbital, and zygomatic nerve. Indications Hay fever, vasomotor rhinitis, dacryorrhea, photophobia, facial pains, disorders and paresthesia of the oral mucosa. Try also in cases of therapy-resistant forms of headache, other results of skull fractures, and maxillary “toothache” in the absence of pathological dental findings. Trigeminal neuralgia, especially where the second branch is affected. Neuralgia of the pterygopalatine ganglion with pain at the interior angle of the eye, the root of the nose, in the nose, in the upper jaw and palate accompanied by attacks of sneezing. For hay fever, it will normally be sufficient to give this injection three times at a few days’ intervals at the start of the pollen season. In vasomotor rhinitis a greater number of injections will normally be required (whenever the symptoms recur). Materials 0.8 mm diameter × (pterygopalatine ganglion) 40 mm or (maxillary nerve) 60–80 mm-long needle. Quantity 1–2 mL. Technique Orientation on the bony skull is essential before the first injection! a. The simplest and safest route for the injection to the pterygopalatine ganglion is from the mouth through the greater palatine foramen. This is located medially from the posterior edge of the second upper molar between the alveolar process and the roof of the palate. The site can be found easily by palpating with a round-ended probe and locating the depression under the mucosa. A quaddle is first set in the mucosa and the 40 mm-long needle is then passed through this along the pterygopalatine canal at an angle of about 60 obliquely in a cranial and dorsal direction, i.e., backward and up. At a depth of about 30 mm the needle is directly next to the pterygopalatine ganglion, where we inject 1–2 mL of procaine solution. The stem of the maxillary nerve is also included, since this is only a few millimeters distant (see Fig. 3.46). Insertion of the needle deeper than 35 mm has to be avoided, because it could end in the orbital cavity and from there through the upper orbital fissure in the middle cranial fossa. Before injecting, we aspirate, because the descending palatine artery runs through this canal as well. A glance at the bony skull will show that this injection cannot create any technical difficulty. What is more, it is absolutely without risk. If the bitter liquid used for the injection into the nasopalatine region runs down, the needle is not lying in the required position in the canal but is too far back and has passed through the soft palate. b. The seated patient leans their head against a headrest. The point of entry is on the upper edge of the zygomatic arch, about midway between the edge of the orbital rim and the ear lobe (see Figs. 3.80, 3.81). The needle is guided in obliquely down toward the front, until it falls into the pterygopalatine fossa at a depth of about 50–60 mm. If the angle of the needle is correct, it will point toward the zygomatic bone on the other side of the skull. When bone contact is obtained, the needle is withdrawn about 1 mm. After aspiration to ascertain that no blood is being drawn into the syringe, 1–2 mL procaine is now injected. The injection is given alternately left and right and, in severe cases, on both sides in a single session. Paresthesia in the region of the side of the nose and upper lip indicates the correct location of the needle. As a sequel of this injection, a harmless hematoma may occasionally occur, which will cause the cheek to become swollen into a “hamster cheek.” If this is noticed early enough, compression with a hard object is indicated, e.g., a metal spatula. No other treatment for this kind of unintentional autohemotherapeutic transfusion is necessary, although it will cause the patient a certain amount of pain during mastication for a few days, because of pressure on the masseter muscle. Fig. 3.80 Injection (b) to the pterygopalatine ganglion. Orientation on the bony skull. Fig. 3.81 Injection (b) to the pterygopalatine ganglion. The needle is inserted above the center of the zygomatic arch, then advanced obliquely toward the pterygopalatine fossa, which lies at a depth of about 60 mm. c. Another injection to the maxillary nerve reaches it more peripherally and is often times sufficient: → injection to the maxillary tuberosity and the maxillary nerve (listed under [c], injection to the maxillary tuberosity and the maxillary nerve, p. 313). 2d. Injection to the Submandibular Ganglion* Anatomy The submandibular ganglion is located next to the lingualis nerve where the nerve turns into the buccal cavity. It is connected to the nerve through two bundles. Sensory lingualis and parasympathetic chorda fibers run through the posterior bundle and the sympathetic root is formed by fibers from the plexus of the facial artery. The anterior bundle brings efferent parasympathetic fibers to the lingualis nerve, which includes the glands of the tongue and the submandibular gland. The submandibular gland receives secretory fibers through special branches of the ganglion. Indications Dry oral mucosa, such as in Sjögren syndrome. Technique The 20 mm-long needle is inserted between the dorsal border of the wisdom tooth and the tongue. We place a 1 mL submucous deposit. We set another 1 mL after infiltrating another 10 mm. This blocks the lingualis nerve, which supplies the frontal part of the tongue. Alternative terminology Injection into the renal bed, injection to the renal pole, perirenal or paranephral injection, splanchnic-nerve or celiac-plexus block. Anatomy The uniform nerve bundle of the major splanchnic nerve (T5 to T6) travels medially and caudally. Combined with the minor splanchnic nerve (T11 to T12) it reaches the abdominal cavity through a fissure in the lumbar part of the diaphragm. Both travel from there to the celiac ganglion. Parts of the larger visceral nerve also run to the suprarenal plexus, parts of the smaller nerve to the renal ganglion. The injection certainly affects other ganglia located in front of or next to the initial part of the abdominal artery, including the aorticorenal ganglia and the mesenteric ganglion. These ganglia that are located on the level of the first lumbar vertebra form the center of the largest autonomic plexus, usually known to laypeople as the solar plexus. From here, all the organs of the upper abdominal area and the small and large intestine up to the left colon bend are regulated sympathetically and parasympathetically, because the vagus nerve joins the plexus as well. Indications 1. Segmental therapy: This injection (see Fig. 3.82) is able to coordinate the functions of the digestive tract, including peristaltic, sphincter function, internal and external secretion, resorption etc., in a manner that no drug can accomplish. Thus, the indication list is a long one: all secretory and motor gastric and intestinal disorders, e.g., gastric and duodenal ulcer with hypo- or hyperacidity, the gastrocardial syndrome, flatulence, epigastric pain of all kinds; acute and chronic disorders affecting the liver (except hepatic carcinoma or abscess and echinococcal cysts), gallbladder (except empyema) and pancreas; pylorospasm in infants, congenital dilatation of the colon (Hirschsprung disease), chronic diarrhea and chronic constipation, circulatory disturbances of the kidney such as eclampsia, anuria; as complementary treatment for diseases of the adrenals and insufficencies after long-term corticosteroid treatment. In the case of shock this injection may be life-saving by loosening up vascular spasms in the splanchnic and renal area. Through the celiac ganglion we can activate the spleen—organ for blood regulation and lymphocyte formation—and, as a result, strengthen the immune system. This injection is also worth trying in multiple sclerosis, in conjunction with injections to the → (T) stellate ganglion. Fig. 3.82 Injection to the celiac plexus. 2. Interference-field search: As a test injection, when an interference field is suspected in the liver, gallbladder, gastric, or kidney regions and that may be due, for example, to hepatitis, gall-bladder disease, gastric ulcer, pancreatitis, dysentery, cholera, typhoid fever, chronic diarrhea, chronic nephropathy, diverticulitis, after abdominal surgery, and the like. Fig. 3.83 Overview of autonomic ganglia and plexus in the abdomen and pelvis. Materials Needle: 80–100 mm 0.8–1.0 mm (thinner needles are too flexible!). Quantity 2–5 mL. Technique a. Original method according to A. W. Vishnevski (1933). In this, the patient is placed on their side on a roll cushion, as for a kidney operation. The 100–120 mm-long needle is inserted at right angles to the skin surface through a quaddle in the angle between the 12th (!) rib and the long extensor (erector spinae) muscle of the back. The needle is advanced under constant plunger pressure through the musculature and the posterior renal fascia into the posterior interfascial space. The neural-therapeutic solution can now flow out of the syringe without meeting any resistance. The large quantity of 150–200 mL 0.25% Novocaine (procaine) solution pushes the anterior and posterior layers of the transverse fascia apart and the product can thus spread in a cranial and caudal direction. It eventually arrives in front of the kidney as far as the renal and supra-renal plexus and beyond as far as the nerve structures of the abdominal organs and especially the sympathetic chain. This method has been used not only for adults but also in children from the second month of life. According to its author, no negative consequences directly attributable to this method were observed. b. We have slightly modified Vishnevski’s method and made it safer. The patient strips to the waist, stands close to a table, and places their trunk flat on it. This is the easiest way for them to relax. If this arrangement is not possible, it will suffice if they support themselves on their fully extended arms in such a way that the trunk is bent slightly forward. If we palpate from the posterior axillary line along the lowest palpable rib (i.e., the 11th rib!) medially towards the spine, we reach the bundle formed by the long extensor muscles of the back, about three finger breadths from the line of the spinous processes. The entry site is in the depression that can be felt there between the lower edge of the 11th rib and the muscle bundle. We set a quaddle over this point, in order to make the entry of the needle painless. The patient does not feel the needle as it is advanced in depth. To make doubly sure, it is advisable first to percuss the lower limit of the lung and mark the injection site two finger breadths below it. Before giving the injection, we ask the patient to breathe deeply in and out and then to hold their breath for a few seconds when we tell them to. With patients who are slow to understand or hard of hearing, this may need to be practiced a few times. We then tell the patient to breathe in deeply and swiftly and painlessly pierce the skin as they do so. The 80–120 mm-long needle tends to bend on entry. This can be avoided by first impaling a sterile swab and using this as a support and guide by holding it between the left thumb and forefinger. Alternatively, the needle may be held near its point in a pair of sterile forceps to guide it through the skin. We now ask the patient to breathe out fully and hold their breath, so that the lower border of the lung will move up as far as possible. The needle is then advanced from its entry point about 30 medially to the sagittal and 60 cranially to the transverse plane, i.e., obliquely rather more up than inward, in the direction of the normal position of the contralateral nipple, only until (depending on the adiposity of the patient), after overcoming the resistance offered by muscle and fascia, one has the distinct sensation at a depth of some 80–100 mm that the needle has penetrated into a void. If one learns to advance the needle under steady pressure on the plunger, it is easy to know when the correct depth is reached since the contents of the syringe then flow out without resistance. The point of the needle is now in the interfascial space near the upper renal pole, in the vicinity of the important suprarenal gland, below the dome of the diaphragm and thus in the neighborhood of the pre-aortic and other ganglia mentioned previously, and of the upper lumbar sympathetic chain. The needle must not be inserted any deeper than that! After aspiration we now inject only about 2 mL and never more than 5 mL. Here, as elsewhere, it is less the quantity that is decisive than the site of the injection! If the injection is not located correctly on the upper kidney pole, we cannot expect it to produce a satisfactory result. In view of the possibility in this case of missing our target at such a depth, it is advisable to repeat the injection once or twice if there is no healing reaction the first time. It is generally best to combine the injection to the major splanchnic nerve with a → (T) preperitoneal injection into the → (T) epigastrium. These two injections should be repeated whenever required, as a rule if there is a recurrence of the symptoms. 3a. Possible Mistakes and Complications with Injections in the Abdominal and Lumbar Regions • If we aspirate arterial blood here, we have gone too deep and too far medially, and have entered the aorta. After the needle is withdrawn slightly, we can inject without risk. Penetration into the aorta is not dangerous, not even the injection into it, and this may be given intentionally for therapeutic purposes in circulatory disturbances affecting the lower extremities. • In connection with injections close to the vertebrae (sympathetic chain, paravertebral, sciatic root anesthesias), we cannot always avoid unintentional penetration of the spinal meninges, spinal dura mater, even when using the correct technique. When exiting the spinal canal, the nerves can be accompanied by leptomeningeal pouches and the needle can penetrate these. For this reason, it is mandatory to aspirate in two directions (twisting the needle 180) before injecting. Direct penetration of the spinal canal is only possible when using a false technique. This type of injection should only be chosen and applied with due caution if simpler measures and less demanding techniques have failed. If liquor is aspirated, the needle went too far medially and entered the spinal canal or it ended up in a liquor pocket of the nerve sheath. The needle is immediately withdrawn and no injection is given at this level on the same day. When using the small amounts of procaine recommended by us, accidental intrathecal injection in the lumbar region would merely produce lumbar anesthesia, which remains without any consequences as soon as it passes off. The specific gravity of liquor is 1.0070, lidocaine is 1.0035, and procaine 1.0055. Hypobaric solutions ascend in the liquor space, hyperbaric descend, and isobaric show the tendency to remain at the location of injection. Again, pressure, concentration, amount, and catabolism of the injection have to be considered. After an unintentional spinal anesthesia, we have the patient lie down with the head in a lowered position. We comfort the patient and assure them that the paralysis will not last long (1–4 hours, depending on the local anesthetic used). The circulatory system has to be monitored. Lumbar anesthesia is not dangerous if the local anesthetic does not contain additives. It needs to be remembered that, due to its speedy resorption and catabolism, procaine acts anesthetically for a shorter period than lidocaine or mepivacaine. Severe headache can be the result of perforation of the nerve root sheath, due to liquor leaking through the puncture opening. The same can happen in the case of lumbar punctures. Entering skin cells, blood, or antiseptics may cause non-bacterial infection. Another cause for severe headache has been found (Krauseneck). The use of combination preparations and local anesthetics from multiple dose vials in the area of the spine should be avoided. Multiple dose vials contain a bacteriostatic additive (methylparaben = methyl p-hydroxybenzoate), which can cause immediate irritation, such as ataxic gait and a tingling sensation in the legs, when entering the nerve root diverticuli. On the day after the injection, the preservatives may cause intoxication symptoms including headache, vertigo, vomiting, nausea, nystagmus, loss of blood pressure, and meningeal syndrome. This is why we use pure local anesthetics without additives only, and refrain from the use of combination preparations for this type of injection. We have to ensure that the ampules that we use for this purpose are also free of bacteriostatic additives. The above-mentioned complications are rather rare and I have not encountered them in 40 years of practice. Nevertheless, they have to be listed in a textbook and their occurrence considered. When following the described protocol, it is almost guaranteed that the irreversible complications reported by Stoehr and Mayer can be avoided. • Perforation of the kidney: The needle can occasionally enter the kidney if the patient has not breathed out, or breathes in again during the injection, if the patient moves suddenly, or if the needle lies too far caudally. One feels an unexpected blunt resistance and the patient reports severe pain at the injection site but which does not radiate. After correcting the position of the needle, one can proceed with the injection. As a rule, perforation of the kidney is of no consequence. According to Spain, 5% of macroscopic hematuria and 3% of renal colics that may occur following perforation of the kidney are “disturbances of a temporary character and require no treatment.” He also states: “Extensive retroperitoneal bleeding might be hard to detect, because in this area, larger amounts of blood can collect without producing any symptoms.” In the countless cases I have treated I have seen only a few hematurias, which passed off without further treatment or any later sequelae after 3 days’ bed rest with an increased fluid intake. Surgeons subject the body to a great deal more when they lay free the kidney, pre-luxate it and split the capsule, not to mention the urologists who intentionally perforate the kidney with a substantially thicker needle during a percutaneous needle biopsy in order to obtain tissue material for their purposes. In both these cases the generally insignificant amount of after-bleeding is accepted as perfectly normal. Compared with the value of this very frequently used injection of ours, the risk is insignificant. To any patient, blood is always an alarm signal. In hematuria, the mixture of blood with urine (and with water in the toilet pan) always suggests a far greater loss of blood than does in fact occur. In my many years of work as medical referee, only three cases have come to my knowledge where a renal hemorrhage has been so severe following accidental perforation that the patient had to be admitted to hospital. It is possible for a massive hematoma in this location to exert pressure on the splanchnic nerves, which can cause symptoms of an adynamic ileus: vomiting and bloated abdomen with lack of peristaltic sounds. F. Huneke published such a case. In 40 years of practice, he saw only a single case in which “a massive hematoma developed several hours after an injection into the renal bed, with severe reflex symptoms, which for a time resembled a peritonitis. Fortunately, no surgery was undertaken. Over the next few days the hematoma was again completely resorbed. The only thing that persisted was the cure of the patient’s previous stomach disorder for which the injection had been given. This kind of thing can neither be foreseen nor avoided, and looked at in the light of day, it is not as tragic as the patient may perhaps feel it to be. The situation described above shows that responsible observation through clinical supervision could be better than the rash decision to perform surgery. In his book Peripheral Nerve Block Pharmacologic by Local Anesthesia, Jenkner wrote: Although perforation or passing a needle through an organ in the abdominal cavity (e.g., also the kidney) seems in theory possible and even necessary, there has never been any negative report on this in publications. For example, in 3000 closely investigated splanchnic-nerve blocks, not a single case of hematuria, peritonitis etc. has been found. Thus, such perforations can produce only minor or unrecognized symptoms. The celiac ganglion should not be blocked in patients whose general condition is poor. This last remark applies to our technique only in a few exceptional cases. Jenkner uses 20–25 mL 0.5% Scandicaine, generally with adrenalin, an additive that we absolutely reject. • If one meets a bony resistance at a depth of 30–40 mm, this is the lateral process. The needle is withdrawn slightly and is then advanced again either above or below the lateral process. If the patient reports a shooting pain down into the legs, we have found a spinal nerve. The position of the needle needs to be corrected by a small amount. If, following an injection into the lumbar sympathetic chain, the patient is temporarily slightly unsteady on his or her legs, this indicates that the motor root fibers have been partially paralyzed. This condition is also harmless and, if procaine has been used, will pass off again within about 30 minutes. • In the injection to the abdominal sympathetic chain, if the wrong technique is used (the needle is too long, the needle is advanced too steeply or inserted too deeply, the patient continues breathing), it may on rare occasions penetrate the diaphragm and reach the lung (if it is inserted too far). In this case the patient coughs immediately and tastes blood, and there will be blood in his or her sputum. This is no cause for concern. Any pneumothorax produced in this way will disappear after a few days without further treatment, provided the patient takes a little care to avoid overexertion. With prior percussion to ascertain the limits of the lung (entry point two finger breadths below), due regard given to the recommendation that immediately before the injection the patient should breathe out and hold their breath, and particularly when remembering not to advance the needle after the myofascial resistance ends and the anesthetic solution flows freely, this will in fact happen only on the very rarest of occasions. Contraindications Apart from extremely rare cases of procaine allergy, there are no contraindications. In such cases we use a preparation based instead on lidocaine (e.g., Xyloneural). In view of the small quantities we use, neither age, a reduced general state of health, nor heart or liver disease are contraindications. With aspiration and a little care, any potentially dangerous complications can be avoided. Nothing much can happen. Anyone who takes the trouble to learn to use this effective weapon will be rewarded a thousandfold for his or her pains. Before testing a patient’s teeth, the patient should be asked whether he or she occasionally has a severe toothache and if so, where. Examination can bring to light fistulas, remains of teeth, lividly discolored areas of mucosa, or atrophic changes after inflammation. No neural therapist should ever omit palpating extraction scars, the zone above the dental roots, and the mucosal sulcus for pressure-sensitive areas. All changes found must be injected in a single session. If palpation of the jaw bone produces localized pain and the local lymph glands are palpable, we have to suspect an odontogenic interference field. In addition, the x-ray picture and vitality tests made by a dentist, together with the electric focal test, are the means available to us for finding the correct injection sites. This is also an appropriate place to remind ourselves that interference fields can be due to the use of several types of metal in the mouth (see p. 91). Difficult as it may be to identify any suspect tooth, the test itself is simple enough. Indications 1. Segmental therapy: In all inflammatory processes in the region of the teeth, mouth, and upper and lower jaws (together with any local treatment that may prove necessary, such as removing the protruding edges of fillings or imperfect crowns, scale, etc.), e.g., acute or chronic periodontitis, alveolitis, post-extraction pain, difficult dentition, paradontopathic conditions, irritation of the dental pulp, slow-healing wounds, ulcerating stomatitis, recurrent aphthuose stomatitis, etc. 2. Interference-field search: As test injection to devitalized, infected, or displaced teeth, alveolar pockets, inflammation due to protruding fillings and along the edge of dental crowns, residual osteitis, foreign bodies, excessively stressed teeth used for anchoring or acting as pillars for dental bridges, closely-set teeth, extraction scars, scars due to root resection and surgery to the maxillary sinuses, parodontosis, residual roots, cysts, gingivitis, stomatitis, etc. Fig. 3.84 Injection to the dental root (from a buccal direction). Fig. 3.85 Injection to the dental root (from a palatinal direction, to the periosteum of the maxilla). Fig. 3.86 Granuloma. Fig. 3.87 Infected residual root. Fig. 3.88 Foreign body. Fig. 3.89 Alveolar pockets. Fig. 3.90 Devitalized teeth. Fig. 3.91 Cyst. Fig. 3.92 Widened periodontal space. Fig. 3.93 Residual osteitis. Fig. 3.94 Displaced tooth. Materials For anesthetizing the injection sites, Gingicaine surface anesthetic (spray) or the Dermo-jet with the dental attachment can be recommended; for injections to the periosteum beyond the alveolar margin, a cartridge or locking syringe is best. If one of these is not available, a size 18 or 20 needle firmly attached to a normal syringe can also be used. Quantity Only about 0.2 mL of procaine solution or Xyloneural from a cartridge ampul is used per injection. Technique a. Injection to the periost above the dental root: We use only 0.2–0.3 mL (infiltrating into the gingiva down to the periost), both palatinally and bucally, for the injection to each dental root to be tested. The injection is given submucously, intramuscularly, and infiltrating all the way down to the periosteum. There is no need to use penicillin, Anthroposan or the like as an additive. In the injection, a millimeter or two may be decisive for the result. The point of the needle should be taken particularly into the hyperalgetic points and into the middle of scar craters following dental extractions. Occasionally, it will sink all the way into the maxillary sinuses without meeting any resistance. This is also an appropriate place to remind the reader of the importance of residual osteitis. When carrying out dental tests, the neural therapist should always include rest periods to enable the patient to assess the results if any and thus localize the culprit within as narrow a range as possible. See Figs. 3.84–3.94 for a selection of possible interference fields in the dental region. b. Intraligamental testing: Another test method, which also reduces the failure rate of the method described under (a), is the intraligamental (intra-aveolar) anesthesia. The local anesthetic is injected into the periodontium (desmodont, vascular connective tissue periosteum that surrounds the dental root inside of the alveola) with a special syringe (e. g., Henke-Ject) that exerts high pressure through a trigger. The injection is almost pain-free. The 0.3 mm needle is put obliquely against the tooth and is advanced 1–2 mm along the tooth. For injections into the distal area of the molars, the needle can be bent and inserted into the fissure with the finger. The solution travels from the dental neck through the circular ligament into the periodontal fissure and from there to the nerve entrance at the apical foramen. The tooth can be extracted immediately (Fig. 3.95). If injection syringes are used for testing, the infiltration has to take place very slowly. Per dental root, one trigger pull of 0.2 mL is sufficient. This requires no less than 20–30 seconds (applying constant pressure)! Only if the testing is done this way can damage to the periodont and loosening of the tooth be ruled out. In cases of periodontitis, this type of testing should not be performed. The idea that instead of injecting several adjacent teeth separately it should be possible to deal with them all together by conduction anesthesia is obvious and tempting, but wrong. For test purposes, conduction anesthesia is unsuitable, because it does not deal with an interference field that may be present. Each tooth must be tested separately, and all suspect teeth need to be tested in a single session! For neural therapy is more than simply “curative anesthesia” or “therapeutic local anesthesia”: the site of the injection is decisive, not the anesthetic effect as such! Fig. 3.95 Intraligamental anesthesia to a molar. Indications: → thyroid disorders, Basedow disease, thyrotoxicosis, hypo- and hyperthyroidism (including “la-tent hyperthyroidism” according to Dosch), goiter, anxiety, a sensation of pressure or of a lump in the throat, heart palpitations, “essential hypertonia,” sleep disorder, menstrual disorders, signs of miscarriage, habitual abortion, menopausal complaints, primarily hot flushes; also try in alopecia, tachycardia or extrasystoles with increasing anxiety; also in fever of unknown etiology, loss of weight and exhaustion with loss of hair, excessive sweating, → neurodystonia, “nervous” abdominal and digestive disorders, increased nervousness and excitability, especially when accompanied by trembling or compulsive weeping, but also when presenting with signs of vegetative overexcitability, including temperature regulation disorders (e. g., increased temperature, flushes, dermatographism, intense sweating, cold feet); hyperemesis gravidarum; in psychological disturbances following castration or in ovarian insufficiency. In acupuncture, these thyroid points are also treated in speakers or singers suffering from hoarseness or loss of voice. In all indications it is irrelevant whether the basic metabolism is normal, increased, or reduced. When an interference field is subjecting the pituitary centers of the diencephalon to stress, this is also frequently accompanied by a substantial reaction of the thyroid. Similarly, due to the mutual relationships within the hormonal system, an interference field in the pelvic region can often also involve the thyroid. A series of injections into the gland, initially weekly and later at longer intervals, will clearly restore the subjective and objective equilibrium in such women. Our thyroid treatment proves to be an effective complementary treatment for → alcoholism. It helps to alleviate the neuro- and psychovegetative side effects of withdrawal (Dietz). It is also helpful in → geriatric disorders. If a thyroiditis has left an interference field, this injection can effect a cure via a lightning reaction. Relative contraindications Radioactive iodine treatment reduces the parenchyma by irradiation. The success of this form of treatment can be assessed only weeks or even months later. During this period, one should refrain from procaine treatment, because the regulating function of the thyroid remains blocked by the radiation damage it has sustained while its effects last. In such cases, the response to the procaine injection may be unusual and can often be paradoxical. In other words, the gland may be over-stimulated to the point of hyperthyroidism. In two cases, thyroiditis occurred, which, however, resolved after about a week. One case was reported to me where an abscess formed following the injection of procaine into a thyroid that had previously been treated with iodine 131. In an attenuated form, this also applies to scintography. If, in such a case, treatment is attempted at all, one should limit oneself to inserting acupuncture needles into the left and right lobes of the thyroid (ST-10), and a third to the dorsal edge of the jugular notch of the sternum (CV-21). We can inject into “cold” or “warm” thyroid nodules only if needle biopsy has excluded a malignant process. Materials The needle should be as fine as possible, about size 18; quantity 0.5–1 mL of 1–2% procaine (without additives!) for each lobe of the gland. Technique It is best to inject the patient when he or she is lying down, since the gland then protrudes forward. The patient is asked to swallow, and the position and size of the thyroid is observed and palpated. The fine needle is inserted quickly and 0.5 mL (never more than 1 mL) of procaine is then injected on each side into the parenchyma of the gland at a depth of 10–20 mm, after checking by aspiration that the point of the needle is not intravasal. This could easily happen, since the thyroid is rich in blood vessels; in such a case, all one need do is to correct the position of the needle and to inject only when it is outside any vessel. If the isthmus is clearly enlarged, a similar quantity is also injected there, but only superficially. If, following the injection, a Horner syndrome is produced, this is a welcome side-effect but indicates too deep an injection. Compulsive weeping may occasionally occur immediately after the injection and indicates that a psychological component of the disorder has been eliminated and that emotional blocks are now being released, to give way to a more balanced state. Injections into the thyroid should initially be repeated at about weekly intervals and, if successful, should then be given at longer intervals determined on an individual basis. Generally, the patient will know by their own observations and state when further treatment is required. In women, treatment of the → (T) pelvic region given in addition will often increase the effectiveness and the result. The interaction between thyroid and ovaries is well known. In cases of hyperthyroidism, repeated → (T) intravenous procaine injections reduce the metabolic rate. We will not always succeed with the attempt to normalize the size and function of the thyroid. We cannot expect success after strumectomy, radiation therapy, and degeneration due to age if sufficient reactive thyroidal tissue is missing. Severe conditions that cannot be helped through conservative treatment have to be treated surgically. According to Pfannenstiel, there is no other surgically treated disorder that shows as many recurrences (8–15%) as strumectomy. This is certainly due to the fact that the symptomatic subtotal thyroid resection cannot eliminate the causes for dysregulation and hyperplasia. These causes include interference fields and chemical regulation blocks, which can inhibit the self-healing mechanisms of the body. Thus, we recommend once again: neural therapy before strumectomy! (See Fig. 3.96 for injection into the thyroid.) Fig. 3.96 Injection into the thyroid. Indications 1. Segmental therapy: In treating chronic tonsillitis, recurrent sore throat and peritonsillar abscesses, sensation of a foreign body in the throat. 2. Interference-field search: As a test injection in patients whose clinical history indicates scarlet fever, diphtheria, frequent or severe sore throat, tonsillar abscesses, tonsillotomy or tonsillectomy. Materials 0.8 mm diameter 80 mm-long needle, short-beveled. If the needle is too short and accidentally comes off the syringe, it may be aspirated or swallowed. Moreover, an unobstructed view of the working area is impossible if the syringe is in the mouth. There is also a special tonsillar test needle according to Strumann, with a plate soldered in position, to prevent the needle from going deeper than 5 mm. Normally it is perfectly possible to dispense with this. Moreover, the soldered connection may melt under frequent hot-air sterilization. In an emergency, it is easy enough to cut off the end of the plastic tube protecting the disposable needle so that the needle projects only 5 mm, thus making it impossible to insert it further when giving the injection. The quantity of solution used for each tonsillar pole is 0.5 mL. Fig. 3.97 Injection to the tonsils. Technique First remove any loosely fitting dental plates and false teeth. If possible, the patient should be seated on a chair with a neck support, or their head should be supported by an assistant or by leaning it back against a wall. This injection should not be attempted if the head cannot be firmly fixed. Good light is another essential prerequisite for good results. The patient should open the mouth wide without sticking the tongue out. The spatula should not be placed too far back, about the middle of the tongue, and should not be pressed down too hard. If the patient is kept distracted by being asked to breathe deeply in and out on command, this will help prevent the undesirable gagging reflex and simplify the physician’s task. It is still easier if the patient pants, i.e., takes continual shallow breaths in and out. If this is still not adequate, we anesthetize the pharyngeal wall with Xylocaine spray and push the base of the tongue aside in a medial direction about level with the wisdom teeth. Particularly for the first test injection, we inject both the upper and lower tonsillar poles, in order to reach as much of the tonsillar tissues with the injection as possible and reduce failures to a minimum. For this the procaine is injected submucously above and below the tonsillar poles. Textbooks caution that, in the case of retrograde blood flow through anastomoses (Bolus injection too fast and paratonsillar infiltration too deep), there is a possibility for the local anesthetic to be pressed through the ascending palatine artery and the external and internal carotid artery into the skull. I have never experienced this scenario. For the injection to the lower poles, the base of the tongue is pressed aside in a medial direction at the end of the alveolar ridge and the anesthetic is then injected submucously between wisdom tooth and the root of the tongue. By this means we prevent tissue damage with the point of the needle, which, in this bacteria-laden tissue, could easily lead to abscess formation. It is perfectly safe to swallow any procaine that runs down. To stimulate the lymphatic flow, we may also set one to three → (T) quaddles in a caudal and medial direction from the mandibular angle. Figure 3.97 shows an injection to the tonsils. If the patient has undergone tonsillectomy, the injection is not given to the tonsillar poles but always into the middle of the scar tissue, directly under the surface of the scar. Sometimes, after the injection under a hard tonsillectomy scar, a Horner syndrome is observed. This is a sign that the numerous autonomic nerve fibers in this area are closely connected with the stellate → (T) ganglion. The injections described above are absolutely without risk and may even be administered to infants (eczema, cradle cap), provided that the most important safety precaution known to us is observed: the injection of any preparation containing procaine into any vessel leading to the brain (cranially from the heart) may in certain circumstances lead to serious complications! This means that before any injection in the region of the head, neck, and the upper portion of the chest, it is essential to aspirate to ascertain that the needle is extra-vasal. If the needle is placed submucously or at least superficially as described, there is no such risk! The carotid artery is all of 40 mm away from the upper, and almost 20 mm from the lower tonsillar poles. But this does not relieve us from the obligation to aspirate briefly before every injection, preferably in two directions (Fig. 3.2). Some patients draw our attention to an autonomic asymmetry, which can be proved objectively from the blood picture (Bergsmann) by telling us that “everything always happens to me on my right (or left) side.” Generally, though not always, the right-hand tonsil acting as an interference field affects the right side of the body, and the left-hand tonsil will affect the left side. Despite this, I always recommend that the first test injection be given bilaterally, to all four tonsillar poles. If there is a positive reaction and if the treatment needs to be repeated, one can then try to make do with an injection only on the side concerned and perhaps by treating only the upper pole. Anatomy The pharyngeal tonsil is located at the roof of the pharynx, toward the posterior wall of the pharynx. Together with the palatine tonsil, the lingual tonsil, and the variable tubal tonsil, they form Waldeyer’s ring (lymphoid ring). Directly in front of it, the pharyngeal hyposphysis is located in newborns. It retards in the course of one’s life. According to Seithel, it remains a consistent structure of 0.33 mm3. It is located at the point of the pharyngeal roof where the anterior lobe of the pituitary gland has developed and consists of adenophypophyseal tissue. Due to the internally secreting nature of the tissue, this scattered cellular lump has the same function (less, based on the smaller mass) as the anterior lobe of the pituitary gland, which is very important for the hormonal control of the vegetative system, for example, through the effects of the adreno-corticotrophic hormone ACTH, which it produces, on the adrenals, spermiogenesis, and follicle maturation. Via endorphin substances, i.e., peptides formed in the brain and the pituitary, the hypophysis is also capable of having a damping effect on the sensation of pain. Indications 1. Adenoids (pharyngeal tonsil) injection: adenoidal proliferation, undulant fever in mouth-breathers when retronasal inflammation is suspected; allergic rhinitis, loss of the sense of smell or taste, inner-ear deafness, and interference field testing of adenotomy scars. 2. Pharyngeal hypophysis injection: Anatomy indicates a supportive role for these injections if we want to affect the pituitary gland (e. g., in addition to injections to the upper → (T) cervical ganglion). Try in inoperable pituitary tumors, pluriglandular dysfunctions, hormonal dysfunction during menopause, diabetes mellitus, and such (Seithel). Materials 0.8 mm diameter × 80 mm-long needle, short-beveled; for the direct injection into the pharyngeal hypophysis, the point of the needle is slightly angled. Quantity 0.5–l mL of procaine solution. Technique a. Adenoids: The needle is inserted without prior mucosal anesthetic above the uvula in the mid-line immediately adjacent to the boundary between hard and soft palate. It is then advanced directly to the posterior wall of the pharynx, until bone contact is made, following which it is withdrawn l mm and the injection given after negative aspiration. b. Pharyngeal hypophysis: To reach the pharyngeal hypophysis, the last 15 mm near the point of the needle must first be bent about 45 degrees with sterile forceps. This facilitates penetration further cranially to the anterior wall of the sphenoidal sinus. If the angled point of the needle is then inserted horizontally as far as its bend and the syringe lowered, the puncture channel is no larger than that described in (a) above. The injection is not particularly painful and absolutely without risk. If the patient has a nosebleed after the injection, he or she should be made to breathe through the nose for about a minute with their head bent back. This should stop the nosebleed. Trigeminal nerve See → (T) nerves (nerve-exit points) (p. 315). Trigeminal nerve root See → (T) Gasserian (otic) ganglion (p. 368). Indications Hypertrophic arthritis of the hip joint, coxitis, coxalgia, spondylitis, ankylosing spondylitis (Bechterew disease). In acupuncture, the trochanter is also needled for sciatica and arthritis of the knee. Fig. 3.98 Injection to the trochanter major. Materials Size 1 needle for slim patients, correspondingly longer for adipose patients. Quantity 2 mL of procaine solution. Technique The trochanter can generally be readily identified on the standing patient, and better still when they are lying down. We insert the needle as far as the periosteum and there inject the 2 ml. The injection should be repeated as required. The effect will then usually increase (see Fig. 3.98). The value of an important discovery is determined only by its usefulness. This is why revealed truth is initially admitted only in private, becomes known more widely only slowly and falteringly, until that which had earlier been stubbornly denied at last seems to be something perfectly natural. Goethe
The Techniques of Neural Therapy
Introduction
Facts to Remember
Symbols Used in the Text
About the Techniques of Neural Therapy
1 Materials
a) Syringes
b) Needles Used in Injections
c) General Equipment Required by the Neural Therapist
d) Accident and Emergency Equipment
2 The Question of Skin Disinfection
3 Procaine (Novocaine), “King of Medicines”
Neural-Therapeutic Preparations Containing Procaine
Neural-Therapeutic Preparations Containing Procaine, with Depot Effect
Neural-Therapeutic Preparations without Procaine
Local Anesthetics with Amide Structure
4 The Question of Dosage
Maximum Doses without Vasoconstrictors
5 Procaine Hypersensitivity and Accidents
Allergy Tests
6 Countermeasures in Accidents
a) Psychogenic Reactions and Mild Collapse
b) Hyperventilation Tetany
c) Allergic Reactions
d) Anaphylactic Shock
e) Poisoning Due to Overdose
f) Shock Conditions
Injection into the Anonymous Vein (Subclavian Vein)
g) Further Precautions and Contraindications
7 Important Rules for Practical Applications
8 Alphabetical List of Injection Techniques
Quantities Stated
Afferent Arteries
Overview
Autohemotherapy***
Cerebrospinal-fluid Pump (CSF Pump) According to Speransky*
Cisternal Procaine Injection According to Reid*
Epidural (Lower) or Caudal Anesthesia***
Epigastrium***
Frankenhäuser’s Ganglia***
Intercostal Nerves**
Intramural Injection into the Uterus**
Intramuscular Infiltration***
Intravenous Procaine Injections***
Joints
Overview
Mastoid Process***
Maxillary Tuberosity and the Maxillary Nerve***
Nasal Conchae*
Nasal spray**
Nerves
1. Nerve-Exit Points on the Head
Overview
2. The Cervical Plexus
Overview
3. Upper Extremities
Overview
4. Lower Extremities
Overview
Paranasal Sinuses*
Paravertebral Anesthesia*
Pelvic Region***
Peridural Anesthesia*
 ).
).
Ponndorf’s Vaccination***
Preperiosteal Infiltration**
Preperitoneal Infiltration**
Presacral Infiltration***
Prostate***
Quaddle Therapy***
Overview
Sacral Foramina (Posterior)*
Scalp***
Scars***
Sciatic Nerve and its Branches***
Overview
Sympathetic Chain and its Ganglia, Parasympathetic Head Ganglia, and Anesthesia of the Celiac Ganglion
Overview
1. Injections to the Sympathetic Chain and its Ganglia***
2. Parasympathetic Ganglia of the Head
3. Injection to the Splanchnic Nerves and the Celiac Ganglion***
Teeth***
Thyroid***
Tonsils
1. Palatine Tonsils***
2. Adenoids (Pharyngeal Tonsil) and Pharyngeal Hypophysis***
Trochanter Major***
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree