Fig. 28.1
The CyberKnife®. A robotic arm points the lightweight 6 MV LINAC to the target. Real-time image guidance based on X-ray imaging (cameras mounted on ceiling, detectors to either side of treatment couch) provides the aiming for this frameless radiosurgery system. Dose conformality and normal tissue protection is provided by firing thin beams from hundreds of different directions, towards the patient
With the CyberKnife system, spinal and paraspinal lesions were initially targeted and tracked on the basis of fiducial markers implanted in vertebrae adjacent to the lesion; this form of targeting has been shown to be accurate to within a mean of 0. 7 mm. A targeting and tracking system based on skeletal features, the Xsight® spine tracking system (Accuray Incorporated, Sunnyvale, CA), has been developed and showed to have a total clinical accuracy to 0. 5–0. 6 mm (Ho et al. 2007). Xsight® employs an X-ray-image-enhancement technique to increase the amount of information provided by the bony anatomy surrounding spinal lesions such that lesions can be targeted and tracked on the basis of spinal anatomy itself, without implanted fiducials. Xsight® registers images taken during treatment to pretreatment (synthetic) images by use of a hierarchical mesh technique, where the calculation is made at a series of discrete points within the region of interest. The result is a deformable registration model that accounts for nonrigid changes in the target position throughout treatment. The deformation can be seen in the middle row of images (under camera image A and B) (Fig. 28.2). Eliminating an invasive procedure (fiducial implantation) from what would otherwise be a noninvasive one significantly improves the patient experience without compromising clinical efficacy.
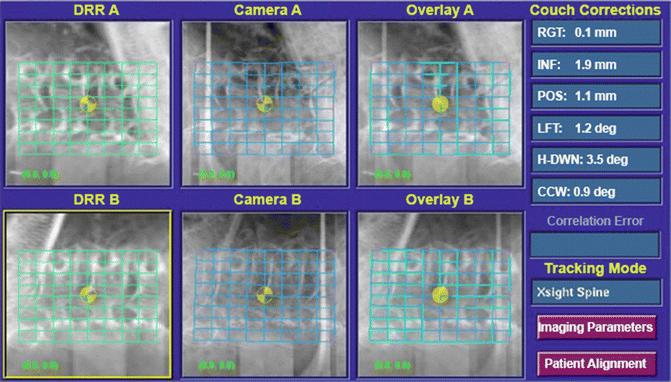

Fig. 28.2
Xsight® spine software registers intra-treatment to pretreatment images by use of a hierarchical mesh technique. In the left column of images, two orthogonal synthetic images obtained from the planning CT scan are displayed. Corresponding live images, taken repeatedly during a session, can be seen in the middle row. Offsets are calculated at a series of discrete points within the region of interest. The result is a deformable registration model that accounts for nonrigid changes in the patient position and between pretreatment and intratreatment imaging. The deformation can be seen in the right column of images. The software calculates errors of patient position in each of the six degrees of motion
Both the CyberKnife and the Novalis® unit emit photons generated by a 6-MV LINAC. The CyberKnife LINAC is mounted on a robotic arm (KUKA® Roboter GmbH, Augsburg, Germany) that can move and point the LINAC with six degrees of freedom. Photons generated by the LINAC pass through circular collimators ranging from 5 to 60 mm in diameter. Recently a dynamic collimator whose diameter may be changed during treatment has been introduced into the clinical practice. The Novalis® LINAC is mounted on a gantry and is equipped with a micromultileaf collimator with 26 leaf pairs. Radiation can be delivered through circular cone arcs or fixed-shape conformal beams via the micromultileaf collimator.
Other technologies have been developed in order to deliver image-guided radiation therapy, which is characterized by enhanced conformality in comparison to conventional radiotherapy (although the treatments are still performed using conventional fractionation protocols). Such systems (Varian® Trilogy, Varian Medical Systems, Palo Alto, CA; Elekta Synergy®, Elekta AB, Stockholm, Sweden; and TomoTherapy®, TomoTherapy Incorporated, Madison, WI) include integrated on-board X-ray imaging, and even CT acquisition, for use during initial patient set-up.
Frameless radiosurgical technology is characterized by the ability to perform hypofractionated treatments. Hypofractionation allows the delivery of ablative, radiosurgical doses to the treated lesion, but allows enhanced protection of the adjacent tissues by delivering the total dose in 2–5 lower-dose fractions and at a lower dose rate (which allows precious time for normal tissues to recover between fractions). Frameless hypofractionated radiosurgery of lesions affecting the cranial nerves and spinal cord substantially enhances treatment safety and prevention of dreaded neurological complications (Romanelli et al. 2006). Frameless systems are not limited to delivering spherical dose distributions (i.e., treatment planning and delivery can be nonisocentric). Early radiosurgical systems treated nonspherical targets by delivering radiation to multiple isocenters packed inside the treated volume. Nonisocentric planning provides a straightforward approach for the treatment of irregularly shaped lesions, and can result in a more homogeneous radiation distribution that is usually very conformal to the shape of the target lesion.
Clinical Applications of Spinal Radiosurgery
Since the initial description of image-guided spinal radiosurgery, a steady increase in published reports has occurred. Selection criteria for spinal radiosurgery are evolving rapidly. In addition, there are reports showing that spinal radiosurgery is a safe treatment for target volumes as large as 200 cm3 (Gerszten et al. 2007); multisession treatments allow the application of SRS to lesions that exceed conventional volume limits. The primary indication for spinal radiosurgery is metastatic cancer in patients who are experiencing pain either owing to disease in vertebrae or because lesions are compressing the spinal cord or nerve roots. Neoplastic lesions (either benign or malignant) are usually quite sensitive to radiation and if proper doses are used, their local growth is arrested. Non-neoplastic lesions such as arteriovenous malformations (AVMs) can also be treated with radiosurgery; such lesions often shrink after irradiation. Radiosurgery does not cause quick decompression of the spinal cord or improve spinal instability and is, therefore, generally contraindicated in the presence of these conditions, although radiosurgical treatment after spinal fixation by kyphoplasty has proven effective (Gerszten et al. 2005b). Of particular note is the fact that radiosurgery can often still be used when conventional radiotherapy has failed (Romanelli and Adler 2008; Romanelli et al. 2006). The available clinical results of spinal radiosurgery are here summarized, paying particular attention to the safety and efficacy of this treatment in relation to specific clinical indications.
Spinal Metastases
Spine is the third most common site of metastatic localization after lung and liver. Spinal metastases are, therefore, a rather common finding in oncological patients, posing a serious challenge to patients and physicians for their tendency to cause severe pain, vertebral instability and spinal cord injuries. The occurrence of spinal metastases in patients with cancer is typically associated with significant morbidity and reduced life expectancy.
Surgery remains the treatment of choice for metastases requiring decompression and vertebral stabilization. Open surgery may achieve the goals of spinal cord decompression, stabilization, pain control, local growth control and histological diagnosis. A posterior approach with laminectomy, however, is sufficient only when the lesion is limited to the posterior elements of the vertebra. In most cases, the massive involvement of the vertebral body would require a full vertebrectomy with instrumented fusion, a complex and bloody procedure, which is offered usually only to a limited number of patients; patients of advanced age, with poor medical condition and disseminated cancer are unable to withstand such surgery.
Image-guided radiosurgery, as well as fractionated conformal radiotherapy, are efficient alternative options for pain relief, neurological function preservation or recovery and control of tumor growth. Radiosurgery, however, as opposed to conventional radiation therapy, provides a fast and highly effective treatment.
Several studies have demonstrated that radiosurgery is safe and effective for the palliation of spinal metastases. CyberKnife image-guided robotic radiosurgery has been widely used to treat spinal metastases, often as the primary treatment modality. At the University of Pittsburgh Medical Center, the largest clinical series to date has been treated with single-fraction radiosurgery (Gerszten et al. 2007). The study involved over 500 lesions in 393 patients with metastases of various (often moderately or highly radioresistant) histologies. Goals of therapy included tumor control, palliation of symptoms, and restoration of neurological function. The average maximum dose to the tumor was 19 Gy delivered in a single session. Sixty-seven patients had not been previously irradiated. In 48 of these cases, a significant decrease in pain was observed during the follow-up period of 6–48 months (median 16 months). Authors reported long-term radiographic control in 88 % of all cases. A remarkable rate of local growth control (up to 100 %) was reported for breast, lung and renal cell carcinoma when radiosurgery was the primary treatment. An overall long-term improvement in pain was obtained in 290 of the 336 cases that presented with pain as a primary indication (86 %).
Similar local control results were found in a series of 58 patients treated at the Georgetown University Hospital for various metastatic lesions with a dose of 21.2 Gy in 3.6 fractions on average (Degen et al. 2005), Most patients were treated for pain rather than other symptoms. Authors reported a rapid and durable pain relief in short-term follow-up. A more extensive cohort of 200 patients was studied by this group (Gagnon et al. 2009). Using a visual analog scale of pain and the SF-12 to assess quality of life (QOL), they found a significant decrease in pain scores over a 4-year follow-up period, no significant change in physical QOL and improvements in the mental component of QOL. Mild and self-limited side effects and no late radiation toxicity were observed.
The CyberKnife was used by Gibbs et al. (2007) for radiosurgery of 74 patients with 102 metastatic lesions. Doses ranged between 16 and 25 Gy in 1–5 fractions. Two-thirds of the patients had been previously irradiated. Symptoms, including pain and neurological deficits, improved in 84 % of cases. Severe myelopathy occurred in three patients after a mean of 7 months from treatment. Two of these patients had received prior irradiation to doses of 50.4 and 39.6 Gy in 1.8-Gy fractions, at 70 and 81 months, respectively, prior to radiosurgery.
Gerszten and coworkers have reported equally good results treating lesions considered as radioresistant, such as renal cell carcinoma (Gerszten et al. 2005a) and melanoma (Gerszten et al. 2006) metastasizing to the spine. In addition, they have advocated a new minimally invasive treatment paradigm for spinal metastatic compression fractures using a combination of kyphoplasty and radiosurgery (Gerszten et al. 2005b). The combination of these two minimally invasive procedures–likely to be a welcome approach for patients dealing with the long-term effects of primary cancer treatment–proved to be safe and effective on short-term (7–20 months) follow-up.
In two papers describing the largest clinical series of metastatic spine lesions treated with the Novalis® system, Ryu et al. (2007) and Jin et al. (2007) reported outcomes of single-fraction radiosurgery in nearly 200 patients. Doses ranged from 8 to 18 Gy, with lower doses chosen when lesions were nearer the spinal cord. Seven-to-nine-beam plans were sufficient to cover tumor volumes while maintaining dose constraints of 10 Gy to less than 10 % of the spinal cord near the lesion. Pain relief was obtained within 4 weeks in 85 % of the 49 patients presenting with pain (Ryu et al. 2007). The duration of pain relief was not reported. A single case of radiation-induced myelopathy was noted in a patient treated 13 months earlier for a breast cancer metastasis to the clivus and C1 (Jin et al. 2007). The patient had been treated with doses that approximated those used to treat patients who suffered no long-term complications. The cervical location of the lesion and prior and post-treatment chemotherapy may have increased the sensitivity of the cord to damage in this patient.
At least one study has shown a relationship between dose and outcome. Pain recurred most commonly in patients receiving less than 14 Gy in a single session (Ryu et al. 2004). Another question that has not been sufficiently answered is whether the two regimens, single versus hypofractionated treatments, differ in terms of efficacy and toxicity. In this context it is noteworthy that the available studies have failed to demonstrate a significant difference in pain control between single and multiple fraction schemes for external beam radiation therapy. For SRS, a retrospective review of the treatment records of 348 metastatic lesions to the spine in 228 consecutive patients treated at University of Pittsburgh and University of Georgetown between January 2000 and 2008 compared single-session to hypofractionated SRS (Heron et al. 2012). One hundred ninety-five lesions were treated using a single-fraction regimen (mean 16.3 Gy), whereas 153 lesions were treated in 3–5 fractions (mean doses of 20.6 Gy in 3 fractions, 23.8 Gy in 4 fractions, and 24.5 Gy in 5 fractions). The primary end point was pain control. Secondary end points included neurological deficit improvement, toxicity, local tumor control, need for retreatment, and overall survival.
Results were interesting and somewhat surprising. Pain control was significantly improved in the single-fraction group for all measured time points up to 1 year post-treatment (100 % vs. 88 %). Rates of toxicity and improvements in neurological deficits were not statistically different. Local tumor control, on the other hand, was significantly better in the hypofractionation group (96 % vs. 70 %, p = 0.001). Similarly, the need for retreatment was significantly lower in this group (1 % vs. 13 %, p < 0.001). One-year overall survival was significantly greater in the hypofractionation than the single-fraction group (63 % vs. 46 %, p = 0.002). Although the retrospective nature of the study precludes strong conclusions, these data suggest that single-fraction radiosurgery provides greater early pain control and equivalent toxicity, whereas hypofractionated treatments achieve greater tumor control lowering the chances of local growth-control failure and consequent need for retreatment in long-term survivors.
Another debated issue is the definition of the target volume in cases where tumor invasion involves only a portion of the vertebra. Patel et al. (2012) retrospectively evaluated differences in clinical outcomes for 154 metastatic lesions in 117 patients with metastatic spine disease treated with a whole versus partial vertebral body contouring approach. Contouring the whole vertebral body reduced the risk of recurrence, improved symptomatic relief and provided improved local tumor control. With the CyberKnife, it is also possible to plan a SRS treatment with two different target volumes (Fig. 28.3), one including the whole vertebra receiving a lower dose (e.g. 8–10 Gy) and a second one including the tumor mass receiving a higher dose (e.g. 16 Gy). This may offer the advantage of treating the whole vertebra, without the necessity to reduce the dose to the tumor.
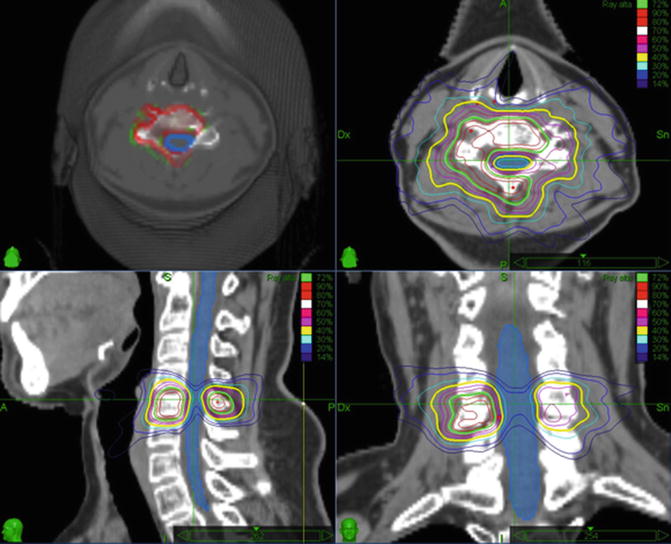

Fig. 28.3
Contours of the entire vertebral body for SRS of metastatic spinal lesions. With the CyberKnife one can plan an SRS treatment with two different target volumes and two different prescribed doses (upper right). One volume, incorporating the whole vertebra, is given a lower dose (the yellow isodose line represents 10 Gy) and a second one, containing only the tumor mass, receives a higher dose (the green isodose line represents 16 Gy). In this manner the whole vertebra can be treated with a dose that can be tolerated by the spinal cord (the light blue line represents the 8 Gy isodose line), while the dose to the tumor remains high
Therefore, available data provide clear answers to important issues, including the efficacy and safety of high conformal radiation to metastatic spine. On the other hand, there are several open questions regarding dose, fractionation, and volume delineation. Radiosurgery can be utilized as a salvage treatment for those with persistent symptoms or radiological progression and who have already undergone external beam radiotherapy. Patients with limited spinal disease, favorable overall performance status, and focal neurological symptoms also benefit from SRS. Control of tumor progression and palliation of symptoms are different goals of treatment, applicable to different degrees in patients with more or less extensive disease. These are issues that must be addressed in a randomized phase III study to ultimately determine whether radiosurgery can be the primary treatment modality for spinal metastases. However the clinical evidence so far available is clearly favorable to the use of spinal radiosurgery to achieve pain relief and local growth control in patients not requiring surgical decompression and/or stabilization or otherwise too sick to be able to tolerate invasive procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








