(1)
Neurological Department, Municipal Hospital Dessau, Dessau-Rosslau, Germany
6.1 High Resolution Ultrasound (HRUS)
6.1.1 Introduction
In 1988, Fornage was the first to introduce the sonography of peripheral nerves [1]. However, with the previously available ultrasound equipment, only the larger pathological alterations such as mass lesions could be detected. In the meantime, all major manufacturers have improved the resolution and quality of their ultrasound images dramatically by means of broad band linear array transducers up to 18 MHz as well as by the introduction of new image processing technologies such as compound imaging and tissue harmonic imaging. Consequently, today, modern techniques facilitate the identification of a number of disorders of the peripheral nerve, even detecting alterations of a single fascicle and its perineurium [2]. At the moment, however, it remains unclear whether additional new techniques like ultrasound contrast agents or elastometric measurements will generate still more information in order to extend further the diagnostic power of this method. Nevertheless, in a recent study it has been demonstrated that contrast enhanced ultrasound (CEUS) seems to be able to detect several perfusion patterns in soft-tissue masses and thus at least to predict malignancy in peripheral nerve sheath tumors [3].
6.1.2 Minimum Technical Requirements
An ultrasound device with a linear broad band transducer up to at least 10 MHz is the minimum technical requirement. With this equipment, an axial resolution of 0.2 mm and a lateral resolution of 0.6 mm can be achieved in the ultrasound image. Ideal are multi-frequency transducers with a wide frequency range from 6 to 18 MHz. Furthermore, the device must have the capability to record various images under different sub-apertures in real time simultaneously. Afterwards, these single frames are mathematically compounded, and, from that, the final image emerges (Compound Imaging). The result is, first, significant noise reduction, and, second, better representation of the tissue boundaries. In addition, the use of tissue harmonic imaging (THI) can improve contrast and lateral resolution, especially in deep situated tissue structures [4]. Moreover, a highly qualitative colour-Doppler function is required to assess the amount of nerve vascularisation. Of course, the examiner should have a good knowledge of the musculoskeletal cross sectional anatomy he intends to display [5].
6.1.3 Examination Technique
Peripheral nerves can be quickly identified by means of anatomical landmarks. Initially, the investigator has to locate anatomical structures such as bones, muscles or blood vessels which lie close to the affected nerve. Once found, from this point, the nerve can simply be traced upwards and downwards in cross sections [5]. For details concerning special nerve entrapments please refer to the ultrasound-imaging sections in Chaps. 8 and 9. Nerves and tendons are sonographically characterized by their anisotropy. For that reason, the optimal echogenicity of a nerve tissue can only be obtained by a strict perpendicular insonation. In order to improve further the image quality of diagnostic ultrasound, particularly in regions with an uneven body surface (e.g. the elbow), the use of aqueous coupling devices is recommended. As peripheral nerves often change their depth in their extremity course, a continuous adjustment of the electronic focus as well as of the transmission frequency of the probe is very important. With increasing transmission frequency (and resolution) the penetration depth decreases, and vice versa. Therefore, for example, the intrapelvic section of the sciatic nerve cannot be examined with high resolution sonography. Magnetic resonance tomography here proves to be superior and is thus an alternative solution. Pathological alterations are always documented in two planes (longitudinally as well as in cross section). Sometimes it may be preferable to record short video sequences. Special questions even require passive movement of the limb to be examined (e.g. snapping triceps syndrome) [5].
6.1.4 Ultrasound Anatomy of the Normal Peripheral Nerve
The cross sectional appearance of the normal peripheral nerve resembles a honeycomb (Fig. 6.1a). Axons and the surrounding endoneurium are arranged in bundles. Some of them form a single fascicle. High resolution ultrasound can resolve and depict single fascicles; however, single axons or the endoneurium are not visible [6]. Depending on the transmission frequency and resolution of the probe, the number of fascicles in the ultrasound image can differ from that determined histologically [7]. In cross sections single fascicles are sonographically characterized by a hypoechoic, tubular appearance. They are embedded in the perineurium and surrounded by the epineurium (or outer layers of perineurium). In contrast to fascicles, epineurium and perineurium show a hyperechoic appearance [6]. Figure 6.1c illustrates these findings. Frequently, it can be difficult to distinguish the very thin epineurium or outer perineurium from the surrounding tissue. In such situations it may help to move the probe upwards or downwards into a different position. In longitudinal sections, the peripheral nerve shows a cable-like course, in contrast to the more fibrillar echotexture of the tendons (Fig. 6.1b). In addition, tendons can be easily distinguished from the nerves sonographically because they always end in a muscle proximally [5, 7]. The most important quantitative parameter in the examination of the peripheral nerve is the measurement of its cross sectional area (CSA) [5]. Sometimes ratios between CSA obtained at different locations within one periphereal nerve or different peripheral nerves are calculated [8]. The measurement should be performed between the border of the hypoechoic fascicles and the hyperechoic epineurium (Fig. 6.1d). If no reference values are available, comparison with the CSA of the healthy side is suggested [5]. In most of the recent studies nerve CSA shows a positive correlation with age and is generally larger in males than in females [9–13]. Moreover is has been published that the nerve CSA was significantly larger in Dutch subjects when compared with Indan subjects. However, further studies are needed to determine the geographical dependency of the CSA. In the meantime it is therefore suggested that laboratories should obtain their own normal values [14]. Please refer to Boehm et al. 2014 and the imaging sections in chapter 8 and 9 to get an overview about published normal values of CSA [9].
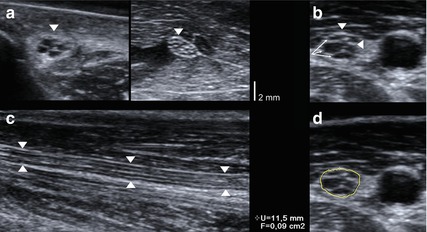

Fig. 6.1
Sonographic findings in the normal peripheral nerve. (a) Honeycomb-like appearance in cross sections. (b) Hypoechoic fascicles (arrowheads), hyperechoic peri- and epineurium (arrows). (c) Cable-like appearance in longitudinal sections. Arrowheads: peripheral nerve. (d) Correct measurement of the cross sectional area between hypoechoic fascicles and hyperechoic epineurium
As described in Sect. 2.2, the nerve starts at the root level with a mono-fascicular architecture, it then subdivides into a fascicular group arrangement and at the end it often demonstrates a multi-fascicular arrangement in the periphery. In parallel to this changing group arrangement, the amount of connective tissue between fascicles (inter fascicular epi- and perineurium) increases. This explains why cervical roots often demonstrate only one individual fascicle in sonography; meanwhile, the number of fascicles, e.g. in the median nerve, increases from proximal to distal. An example is shown in Fig. 6.2.
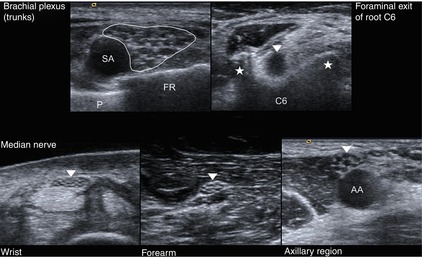

Fig. 6.2
Different sonographical appearance of the peripheral nerve depending on the anatomical localisation. Upper part, left: SA subclavian artery, P pleura, FR first rib. Upper part, right: arrowhead – root C6, asterisks – tubercula of the transverse processus of C6. Lower part: arrowheads – median nerve at different anatomical positions
With the aid of colour flow Doppler functions of the current ultrasound equipment, the device is now able to identify the normal epineural vessels more frequently. However, excessive vascularisation (“hypervascularisation”) is considered as non-physiological by some authors [15–17]. In a few conditions (e.g. entrapment syndromes) attempts have recently been made to quantify these changes [16, 17]. Due to lack of adequate studies and diagnostic criteria, we recommend to simply compare and record the vascularisation of the affected and the unaffected side qualitatively (Fig. 6.3). However, it has at least been shown that an increased intraneural vascularisation is associated with axonal loss in ulnar neuropathy cases at the elbow [17].
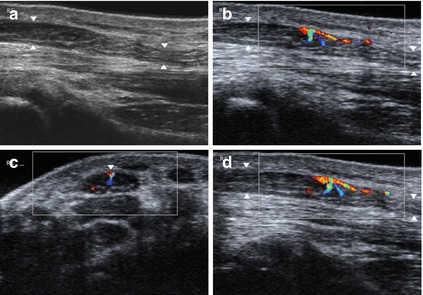

Fig. 6.3
Pathological vascularisation of the epineural vessels affecting the median nerve (arrowheads) proximal to entrapment (carpal tunnel syndrome). (a) Grayscale image. (b–d) Color-flow Doppler images showing the abnormal vascularisation in cross section (c) and longitudinal sections (b, d)
Another novel approach to detect abnormalities is assessing the echogenicity of nerve cross sections quantitatively [18, 19]. Thereby, quantification of nerve density – defined as the ratio between hypoechoic and hyperechoic areas/pixels of peripheral nerves in the ultrasound image – is capable of discriminating between normal and pathological nerves in carpal tunnel syndromes, respectively, between mild and severe CTS cases [19]. However, until now it remains unclear whether this method will be accepted in the clinical routine.
6.1.5 Application to Entrapment Neuropathies
If a peripheral nerve is externally compressed or entrapped by ligaments, bone fragments, callus or other pathological conditions, it always responds with the same morphological alteration. At the site of compression, an abrupt flattening occurs with a decrease of the cross sectional area and a decreased nerve diameter in longitudinal sections (Fig. 6.4b). Proximally and sometimes distally to the site of compression, a segmental swelling of the nerve can be observed and, consequently, the cross sectional area and/or nerve diameter increases within that nerve segment. Furthermore, in such cross sections, a typical hypoechoic swelling of the fascicles appears. Later on, the fascicles are effaced due to the increasing oedema and the honeycomb-like echotexture is replaced by a homogeneous hypoechoic one [20–23]. This phenomenon is sometimes falsely referred to as “pseudoneuroma”. If a distal nerve-swelling is also present, e.g. in advanced stages of carpal tunnel syndrome, the nerve demonstrates an hour-glass-like appearance (Fig. 6.4a, c). Actually, the cause of increased size and loss of echogenicity of compressed nerves is not well understood. Some authors discuss an increased vascularity of the nerve proximal to the site of entrapment [22]. As mentioned above, this has been demonstrated using colour flow Doppler [15–17] (Fig. 6.3). After treatment of carpal tunnel syndrome with steroid injections, nerve swelling and vascularity as well as echogenicity decreased significantly [23]. Sometimes a blurred demarcation of the outer epineurium of the enlarged nerve segments can be observed, probably caused by an epineuritis, which may turn into a fibrosis of the epineurium later on. This reaction will be recognisable by a thickening of the hyperechoic epineurium on ultrasound [21] (Fig. 6.4c). According to our own experience, these typical signs may also be seen in conditions after nerve surgery, and, of course, following nerve injuries. Furthermore, it was reported that a thickened outer epineurium is a sonographic sign suggesting snapping ulnar nerve syndrome [24]. As a consequence of passive displacement of peripheral nerves in late stages of entrapment, the proximally thickened nerve segment may slowly develop signs of a reactive perineural fibrosis, a real pseudoneuroma. We have observed hyperechogenic spots within proximally enlarged hypoechoic nerve segments in patients with severe carpal tunnel syndrome. They may probably reflect the perineural fibrosis but a replacement of nerve tissue by fat after axonal loss is also conceivable (Fig. 6.4c). Interestingly, also in these late stages, proximal cut-off values of median nerve cross sections remain enlarged, and thus do not reflect signs of secondary nerve atrophy in the majority of cases [25].
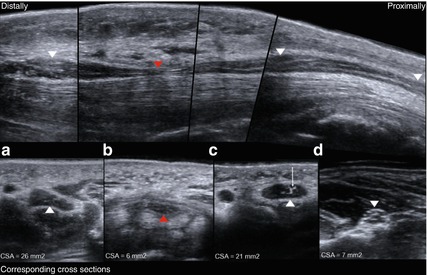

Fig. 6.4
Typical sonographical findings in an entrapment neuropathy. Upper part: longitudinal reconstruction of the median nerve at the wrist (carpal tunnel). Lower part: corresponding cross sections. White arrowheads: median nerve distal and proximal to the site of compression. Red arrowheads: median nerve at the site of compression. (d) Unaffected median nerve at the forearm with normal cross sectional area and honeycomb-like echotexture. (c) Proximal nerve swelling with hypoechoic fascicular effacement, oedema and hyperechoic epineural thickening as well as increased cross sectional area. Small arrow: suspected perineural fibrosis. (b) At the site of compression abrupt flattening of the median nerve (red arrowhead) with decreased cross sectional area. (a) Distal nerve swelling with similar changes as described in (b)
In addition to these qualitative alterations characterizing nerve entrapment, quantitative measurements of different cross sectional areas have been introduced as the most commonly used parameter. One possible opportunity is related to the use of cut-off values at the site of the proximal nerve swelling. On the other hand, calculation of a ratio between the cross sectional area at the site of proximal nerve swelling and the cross sectional area which we obtain more proximally, where the nerve is in a healthy condition, is quite a reliable practice. These ratios are referred to as “wrist to forearm ratio” in carpal tunnel syndrome and “cubital to humeral ratio” in ulnar neuropathy at the elbow [26–30]. However, flattening ratios of nerve diameter, amount of bowing of the flexor retinaculum, as well as characteristics of nerve mobility and echogenicity have been used infrequently [31].
Former systematic meta-analyses of clinical trials with measurements of the cross sectional area in carpal tunnel syndrome have evaluated inferiority compared with electrodiagnostic testing [32–34]. In contrast, the American Association of Neuromuscular and Electrodiagnostic Medicine recently published a systematic evidence-based review regarding the potential value of high resolution ultrasound as a “first line tool” in the diagnosis of carpal tunnel syndrome. Considering a total of 45 studies concerning sensitivity and specificity of HRUS compared to electrodiagnostic testing (4 of them had evidence-class I), HRUS was assigned recommendation level A. Moreover, HRUS for detection of structural and anatomical abnormalities in carpal tunnel syndrome was assigned recommendation level B after considering a total of 23 studies [35]. For more details (cut-off values or ratios in median nerve) please refer to Chap. 8. Unfortunately, the results derived from these studies concerning the correlation of cross sectional area measures with electrodiagnostic severity in carpal tunnel syndrome remain contradictory. Some investigators have found a strong correlation [36, 37], whereas others did not [38–40]. Concerning the ulnar neuropathy at the elbow, systematic evidence-based recommendations or systematic meta-analyses are currently missing. However, the most recent report still stated that CSA and swelling ratio indeed seem to have comparable diagnostic values [30, 41].
In summary, sensitivity and specificity obtained by high resolution ultrasound and electrodiagnostic testing in nerve entrapment do not differ significantly. However, only classical nerve conduction studies allow to asses the severity of a nerve entrapment, which means differentiating between demyelinising and axonal lesion. Furthermore, until now there has been a lack of evidence-based recommendations concerning ulnar neuropathy at the elbow [35, 39, 42].
In some individual cases (patients who refuse electrodiagnostic testing, children, patients with different overlapping conditions such as nerve entrapment and any kind of a generalized neuropathy, and patients in advanced stage of entrapment with axonal loss in whom the results of electrodiagnostic testing are ambiguous), HRUS may be used solely for assessment of nerve entrapment, taking into account the qualitative and quantitative changes mentioned above [5, 43, 44]. The remaining majority of patients should continue undergoing nerve conduction studies as the first-line test. Nevertheless, HRUS can provide additional information that may influence the diagnostic and therapeutic approach.
The median nerve in particular can present a couple of anatomical variations (high division, accessory branches, persistent median artery, etc.) in approximately 33 % of the symptomatic individuals [2, 26, 45]. High division of the median nerve is probably not a risk factor in developing a carpal tunnel syndrome and was observed in 14.4 % of healthy subjects and in 18.5 % of symptomatic patients [45]. Even small nerve branches such as the palmar cutaneous branch of the median nerve and its variations may be depicted [46]. Some might hold an increased risk of injury, particularly when modern endoscopic carpal tunnel release is applied. HRUS can detect dangerous anomalies before intervention [47, 48]. An example is shown in Fig. 6.5a.
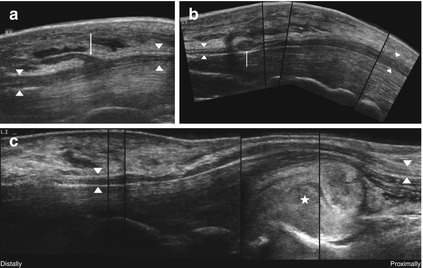

Fig. 6.5
HRUS, anatomical variations and secondary causes of nerve compression in the median nerve (arrowheads) at the level of carpal tunnel. (a) Transligamentous running branch supplying the thenar-muscles (arrow). (b) Small schwannoma (arrow) within the carpal tunnel. (c) Acute bleeding (asterisk) in a patient undergoing anticoagulant therapy
Additionally, there are a lot of space-occupying lesions in the carpal tunnel that cause nerve compression (extraneural or intraneural ganglion cysts, which are described in Sect. 13.3.8, tumors of the peripheral nerve sheath, bone fragments, thrombosis of median artery, tendinitis, accessory muscles, etc.). Some require a different therapeutic approach (Fig. 6.5b, c). In these cases too, HRUS provides reliable information before surgery [2, 26, 49, 50].
Ulnar neuropathy at the elbow is sometimes characterized by two or even more compression mechanisms (ancoaneus epitrochlearis muscle, Struther’s ligament, subluxation or luxation tendency, etc.). In a recent study, subluxation occurred in 14 % and luxation in 6.7 %, whereas in healthy controls only 5.7 % had either subluxation or luxation of the ulnar nerve [51]. According to another report, the values for subluxation and luxation were similar. A prevalence of accessory muscle was found to be 8.8 % among patients with ulnar neuropathy. Furthermore, secondary causes of compression (osteophytes, ganglionic cyst, osseous fragment and other post-traumatic lesions) have been observed in 12.1 % of cases [52]. The detection of post-traumatic lesions with multiple and/or atypical sites of entrapment as well as cubitus varus deformity is particularly important because they have an influence on the surgical approach. Therefore, HRUS provides a fast and cost-effective way to perform the required surgery [53, 54]. For images and further details please refer to Chap. 8 (ulnar nerve).
If surgical intervention fails, e.g. after carpal tunnel release, high resolution HRUS will evaluate exactly the pitfalls (remaining stenosis caused by incomplete flexor retinaculum release or scar tissue, major nerve injury or even misleading preoperative diagnosis) [55–57]. However, a remaining reduction of median nerve cross sectional area after carpal tunnel release is at present still of limited value in the assessment of poor outcomes [58, 59]. An example is shown in Chap. 8 (median nerve).
Finally, ultrasound guided injection of corticosteroids in patients with ulnar neuropathy at the elbow as well as sonographically guided percutaneous needle release of the carpal tunnel may represent an alternative to the standard surgical approach in future [60, 61].
Unfortunately, there are only case reports or small case series regarding rare entrapment neuropathies such as tarsal tunnel syndrome, Guyon’s canal syndrome, etc. Because of the lack of systematic studies, only normal values and some cut-off values but no CSA-ratios are available at the present. Diagnosis should be based on the qualitative characteristics of nerve entrapment mentioned above and on the published normal values of nerve cross sectional areas. Thus, nerve conduction studies in conjunction with needle electromyography still remain the first line tool within the diagnostic pathway. Similar to the more common entrapment neuropathies, HRUS can again demonstrate special features such as ganglion cysts in the tarsal tunnel or in the Guyon’s canal as well as fibrous ligaments of the long peroneal muscle in case of peroneal nerve entrapment [21, 62]. Please refer to Chaps. 8 and 9 for additional details.
In summary, the diagnostic value of high resolution ultrasound in nerve entrapment mainly consists, first, of detection of anatomical anomalies and, second, of the evaluation of special pitfalls.
6.1.6 Application to Focal Traumatic Nerve Lesions
As previously mentioned, there are different degrees of severity of a nerve injury. For a detailed description please refer to Sects. 3.1 and 3.2. The Sunderland classification is based on the morphological changes of the injured nerve, and it is therefore the most appropriate to describe the corresponding sonomorphological alterations of a nerve injury [63]. As mentioned above, high resolution ultrasound can depict single fascicles, the perineurium and the epineurium of a peripheral nerve; however, it cannot resolve a single axon, its myelin-sheath and the corresponding endoneurium. Hence, a sonographical differentiation between grade I, II and III injuries is not possible. This restriction has less significance because, in practice, we need to distinguish between the minor lesions capable of spontaneous recovery, where surgical intervention is hardly needed, and the major lesions, where surgical treatment is almost always required. In the high resolution ultrasound related literature, the term “minor lesion” is applied to grade I–III lesions in accordance with the Sunderland stratification, whereas the term “major lesion” corresponds to a grade IV or V injury [64–66].
Minor lesions create no or only subtle changes on ultrasound. According to our experience the normal echotexture (honeycomb) below, above and at the site of injury is preserved. In the case of demyelination (grade I), either no alteration or a focal hypoechoic edema of fascicles and a consecutive segmental enlargement similar to entrapment neuropathies may be observed. If an axonal loss occurred with preserved perineurium (grades II and III, loss of axon continuity without or with injury to the endoneurium) the entire nerve segment, especially distal to the injury, will show hypoechoic enlarged fascicles and nerve swelling (Fig. 6.6a). However, mostly these subtle alterations are only recognizable if compared with the unaffected side [65]. In accordance with the latest studies performed by MR neurography in rats with traction injuries, the nerve enlargement and oedema will probably reflect the Wallerian degeneration; it may partly disappear when nerve regeneration takes place [66]. In grades II to III, continuity of the epineurium is always preserved. A pseudoneuroma due to intraneural connective tissue fibrosis may occur weeks later, but not a real neuroma formation [67].
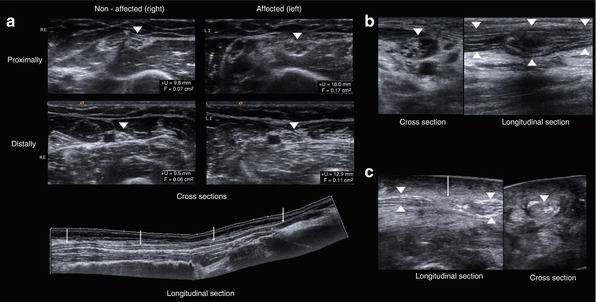

Fig. 6.6
Minor and major lesions following a nerve injury in HRUS. (a) Minor lesion (II/III according to SUNDERLAND) presenting an oedema of the fascicles and an enlargement of the entire nerve segment especially distal to the lesion after traction injury of ulnar nerve and compared with the unaffected side. The normal honeycomb-like appearance of the nerve in cross sections is preserved. Arrowheads – ulnar nerve. Arrows – decreasing oedema from proximal to distal. (b) Type IV lesion with a partly destroyed normal echotexture of the nerve (arrowheads) in cross sections and the cable-like appearance in longitudinal sections at the site of the injury; however, the continuity of the epineurium is preserved. (c) Type V lesion with interrupted continuity (arrow) of the peripheral nerve (arrowheads), complete destruction of the normal echotexture in cross sections at the site of the injury and retracted nerve stumps
However, major lesions always create distinct sonographic changes regardless of the time elapsed after injury. Grade IV lesions with preserved epineurium continuity are characterized by a partial or complete loss of the normal echotexture (honeycomb-like) at the site of the injury; it reflects the advanced destruction of fascicles and perineurium. In early stages, intraneural bleeding can sometimes be observed. Further characteristics – quite similar to grade II to III lesions and due to Wallerian degeneration – are mainly to be found in the nerve segment distally to the lesion. An additionally disrupted continuity of the epineurium with retracted nerve stumps indicates a grade V lesion (Fig. 6.6b, c). This kind of damage is easy to evaluate [64–66, 68, 69]. In the case of a later stage of a major lesion without any previous surgical treatment, HRUS can demonstrate a neuroma in continuity (in a grade IV lesion) or even a stump neuroma (then indicating a grade V lesion). A neuroma consists of nerve fibers regenerating from proximal and intraneural scar tissue which stops the sprouting process. Due to disrupted continuity of the peri- and/or endoneurium, and to severe reactive fibrosis, the newly sprouting nerve fibers are unable to find their way down to the target and thus will form a neuroma [70]. Neuromas sonographically appear as a globose, ovoid, fusiform and sometimes irregular, rather hypoechoic mass and have well-defined margins. The fascicles of the proximal nerve segment often enter the neuroma in a chaotic manner and disappear within it. Sometimes neuromas demonstrate an increased vascularisation. As mentioned, they are located either within the continuity of a nerve trunk or at the end of the proximal nerve stump [64–66, 68, 69]. Examples are given in Fig. 6.7a, b. The differentiation with respect to smaller tumors of the peripheral nerve sheath can be challenging. This applies especially to a neuroma in continuity. Here, a detailed anamnesis survey must be used. If the history of a suitable injury is reported, it becomes more likely that the mass will not be a peripheral nerve sheath tumor.
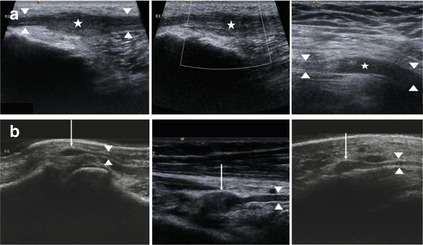

Fig. 6.7
Neuroma formation in HRUS. (a) Grade IV lesion with neuroma in continuity (asterisk) of the common peroneal nerve (left) and the femoral nerve (right). Arrowheads: nerve in continuity. Note the absent vascularisation. (b) Several grade V lesions with stump neuroma arising from the proximal nerve stump (arrow)
As a complementary method, and particularly if deterioration or loss of nerve function is trauma related, high resolution sonography provides several important advantages in comparison to mere electrodiagnostic testing. First, the therapeutic relevant major lesions can be detected very early with an accuracy of 93 %, whereas electrodiagnostic testing alone provides an accurate statement whether reinnervation takes place (minor lesion) or fails (major lesion) still rather late [68]. By means of electrodiagnosis only, such an measurement is still obvious after a period of 2 or 3 months. However, patients with major lesions will surely benefit from earlier surgical treatment because the progressing atrophy of the target organs (“time is muscle”) can be stopped earlier [65, 66]. Second, in contrast to electrodiagnostic testing, high resolution sonography allows the exact localisation of the involved nerve segment [71]. This fact is especially important if traction injuries have occurred which might have affected the nerve at various points. For instance, electromyography studies cannot distinguish between a single and multiple serial lesions. Regardless, the extent of the electromyographic examination that is sometimes unpleasant to patients can thereby often be significantly reduced. Moreover, it is important that exact lesion localization helps to minimize surgical time and trauma.
Furthermore, HRUS can provide extremely helpful information on detecting the cause of postoperatively continuous and painful nerve irritation (e.g. scar tissue, bone fragments, callus, or pseudarthrosis in the neighborhood), and provides detailed answers to questions regarding the failure of electrodiagnostic testing. Finally, after nerve repair, mostly by means of nerve grafting, HRUS can depict the site of the fascicle coaptation. Surgical suture material appears as a hyperechoic nodule. At the site of the nerve adaptation, the development of a neuroma can re-occur and disturb axon sprouting. Rapid growth of these “suture neuromas” will result in an unfavorable outcome regarding functional nerve recovery, so the surgeon has to consider re-suturing [64–66, 68, 69, 71]. Figure 6.8a–e illustrates some of the above-mentioned conditions.
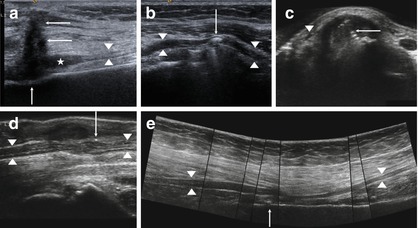

Fig. 6.8
Continuous post-traumatic nerve irritations in HRUS. (a) Scarring (arrow) in front of a stump neuroma (asterisk) of the superficial peroneal nerve (arrowheads) after chainsaw injury. (b) Cerclage (arrow) compressing the common radial nerve (arrowheads) at the upper arm. (c) Ostheosynthesis (arrow) affecting the tendons and the superficial radial nerve (arrowhead). (d) Artefact by sutures (arrow) after surgery of the median nerve (arrowheads). (e) Sciatic nerve (arrowheads) is compressed due to cerclage and reactive scarring (arrow)
In several surgical institutions the high resolution ultrasound is also applied intra-operatively. The short distance between probe and nerve allows examination with the highest possible resolution. Thereby, important details like the rate of epineural or perineural fibrosis can be assessed which acts as an aid to differentiate the type of surgery required, microsurgical neurolysis or segment resection and grafting, or even a combination of both methods applied on separate nerve sectors [5, 72].
6.1.7 Application to Ganglion Cysts, Nerve-Sheath-Tumors, and Other Intraneural Space-Occupying Lesions
As mentioned in Sect. 6.1.5, a peripheral nerve can be externally compressed or entrapped by a couple of soft tissue space-occupying lesions. With regard to surgical aspects of nerve tumors or tumor-like conditions, please refer to Chap. 13. Nevertheless, in order to offer complete continuity in our presentation of the principles of sonographic imaging and, furthermore, in order to give a comparative survey of imaging of all kinds of focal neuropathies (entrapment, traumatic lesions in continuity of different degrees, pseudoneuroma, neuroma, neoplasms, inflammation pathologies), we continue with tumors. Surgical aspects will be presented in Chaps. 11 and 13.
According to our experience, when we apply high resolution sonography to special focal neuropathies which are tumor related, extraneurally located ganglion cysts are the most common space-occupying lesions we observe. From this point of view we want to describe briefly their typical sonographical findings here, although they are more often located outside the peripheral nerve. Ganglion cysts can arise from a joint or from a tendon sheath [73]. The appearance of ganglion cysts ranges from either simple to complex. A simple ganglion cyst is mono-locular and characterized by an anechoic internal echotexture with a thin and well-defined margin, an absent vascularisation in colour Doppler, and a variable post-acoustic enhancement. Complex ganglion cysts may show septations, internal hyperechoic reflections as well as an irregular shape. They are usually larger than simple ganglion cysts. Echotexture may be hypoechoic, but sometimes with a partially solid component. Some show a colour Doppler flow, and nearly all have a post-acoustic enhancement [74–76]. Occasionally the communication with the joint or tendon is visible [74]. Since ganglion cysts have infrequent vascularisation, they may be differentiated by this fact from other mass lesions [74–76]. An overview of typical sonographical findings is presented in Fig. 6.9.
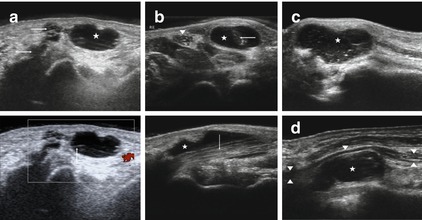

Fig. 6.9
Typical sonographical representation of ganglion cysts. (a) Ganglion cyst (asterisk) arising from the basic joint of the thumb (small arrows). Note the post acoustic enhancement. No Vascularization was not detected with the aid of colour Doppler. (b) Ganglion cyst (asterisk) arising from the tendon of the flexor carpi radialis muscle (arrow), arrowhead: median nerve. (c) Complex ganglion cysts with septations as well as internal reflections (asterisk). (d) Ganglion cyst (asterisk) compressing the ulnar nerve (arrowheads) at Guyon’s canal
Among the different types of benign soft tissue tumors only 10 % originate from the peripheral nerve structure (nerve trunks, plexus or roots). They are also referred to as benign tumors of the peripheral nerve sheath. The most common are the schwannoma (synonym: neurinoma) and the neurofibroma [77]. A solid schwannoma usually affects a single fascicle only, whereas a solid neurofibroma arises from a fascicle group, a fact that helps to explain why schwannomas are easier to treat surgically as described in Sect. 12.3.1. Both mass lesions are characterized by a slow growth over years or decades. Therefore, neurological abnormalities appear very late. As an early symptom, a positive Hoffmann-Tinel sign and irradiating pain provoked during pressure at the site of the space-occupying lesion is noted. In neurofibromatosis type I multiple solid neurofibromas within a peripheral nerve may be observed. On the other hand, the rarely occurring plexiform neurofibroma is a special form of multiple manifestations of neurofibromas within one nerve trunk. Due to its net-like growth, gradually all fascicles are involved. Subsequently, early development of a sensorimotor failure followed by axonal loss as well as other severe neurological symptoms, particularly pain, is common in plexiform manifestations [78, 79]. Moreover, in patients with neurofibromatosis type 1, the risk of malignant transformation is enlarged [80]. The rare perineurioma shows a similar type of growth within a peripheral nerve [78, 79].
The typical songraphical presentation of solid schwannomas or neurofibromas is characterized by a peripheral nerve that centrically or eccentrically enters and exits a rather homogeneous hypoechoic ovoid or fusiform mass which has well defined margins and a post acoustic enhancement (Fig. 6.10a). In larger solitary neurofibromas and schwannomas, good vascularization may be observed with the aid of color-flow Doppler. Timely progressed schwannomas in particular form additional regressive signs such as hypoechoic cysts, calcifications, and larger hyperechoic areas [81, 82]. This is demonstrated in Fig. 6.10b–d. However, in a recent report it has been shown that a differentiation between schwannoma and neurofibroma using HRUS is impossible. A more eccentrical growth may suggest a schwannoma [83].
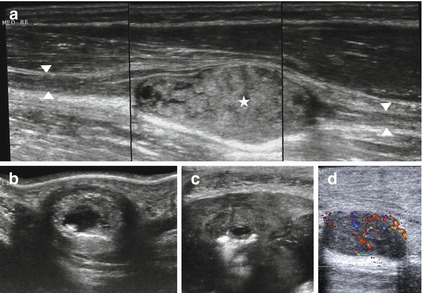

Fig. 6.10
Typical sonographical representation of a benign tumor of the peripheral nerve sheath. (a) Median nerve (arrowheads) enters and exits a schwannoma (asterisk) at the level of the forearm. Note the post acoustic enhancement. (b, c) Regressive changes in late shwannomas like cysts and areas of hyperechogenicity. (d) Well vascularized tumor of the peripheral nerve sheath
Quite another severe challenge arises when differentiation between a plexiform growing tumor, a tumor-like lesion (see Sects. 13.3.3 and 13.3.4, and Chap. 14) or a focal manifestation of an immune-neuropathy (see Chap. 10) is sonographically required [84] (Fig. 6.11a). According to our experience, all of these completely different focal lesions can show a focal fusiform enlargement of the nerve and a hypertrophic-hypoechoic remodelling of its fascicles. For further detailed information on the various inflammatory nerve diseases that we have to take into account when different treatment modalities are up for decision, refer again to Chap. 10. In the case of a plexiform neurofibroma, a history of a neurocutaneous syndrome (neurofibromatosis type I) is often but not always present, whereas in an inflammatory neuropathy, the fact that different nerves are clinically, subclinically (in EDX!), and sonographically affected can help. Finally, the more significant contrast agent uptake of a tumor in MR (magnetic resonance) neurography may support the differential diagnosis. However, sometimes a biopsy of nerve fascicle leads to a definitive diagnosis [84, 85].
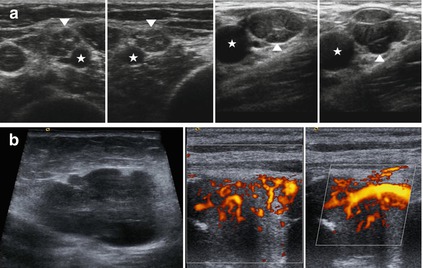

Fig. 6.11
Challenging differentiation between plexiform growing tumors of the peripheral nerve sheath (perineurioma, neurofibroma) and other neuropathies in HRUS. Malign tumors of the peripheral nerve sheath. (a) Left – hypoechoic swelling of the fascicles of the median nerve (arrowheads) in a case of Parsonage Turner syndrome at the upper arm. Asterisk shows the brachial artery. Right – perineurioma of the ulnar nerve at the axilla (arrowhead) shows a similar appearance. Asterisk: axillary artery. (b) Sonographical representation of a malign tumor of the peripheral nerve sheath. Note the anarchic vascular pattern, the irregular margins and the infiltrative growth
Furthermore we may be very rarely confronted with a perineurioma which shows similar sonographical appearance as plexiform neurofibroma. On the other hand the very rare granular cell tumor presents similar to a late case of schwannoma with internal calcifications but irregular borders [82]. However, these are rarities.
Malignant tumors of the peripheral nerve sheath are equally rare. They are characterized by fast growth within some weeks or months and an early incidence of neurological abnormalities [78, 79]. Sonographically, an infiltrative growth, an irregular tumor surface, and an inhomogeneous echotexture are observed. The criteria mentioned above yield a sensitivity of 82%, but the specificity is only 38 % in HRUS. If the mass demonstrates good vascularisation and further characteristics such as anarchic vascular patterns, vessel trifurcations, vessel occlusions and stenosis, these findings can help to improve the sensitivity and specificity [86] (Fig. 6.11b). It has recently been made evident that contrast enhanced ultrasound (CEUS) enables differentiation between benign and malign soft tissue masses. Therefore, the type of perfusion pattern after administration of ultrasound contrast agent was used. The combination of three features (first, perfusion pattern of the mass either inhomogeneous or perfusion at the margins only, second, size >3.3 cm, and third, location below the superficial fascia) yields a sensitivity of 89 % and a specificity of 85 % as well as a positive predictive value concerning a malignancy of 86 % [3].
Among intraneural space-occupying lesions which do not originate from nerve tissue itself are rarities such as lipomas, fibrolipomas, haemangiomas [87–90] and tumor like lesions such as amyolidoma, lymphoma, sarcoidosis-lymphoma syndrome, leprosy or extramedullary affection of the peripheral nerve due to plasmacytoma. We will briefly discuss some of these in Chap. 14.
These lesions also include the uncommon solitary or multiple intraneural ganglion cysts. The peroneal nerve near the tibio-fibular joint is commonly affected, but several different locations in the neighbourhood of joints come into consideration such as, the tibial nerve in the tarsal tunnel. Due to degenerative and destructive alterations of joints, e.g. of the tibio-fibular or ankle joints, a dissection of the epineurium of the nerve branch supplying them occurs. As a consequence, a retrograde filling of the nerve with synovia is assumed [91]. Commonly, the patients suffer pain. Fluctuating weakness is more frequent [92]. Multicystic intraneural manifestations are then frequently to be detected sonographically, and they are characterized by a hypoechoic and string of pearls-like enlargement of the entire affected nerve segment. The fascicles and the perineurium can be clearly identified as “floating” or “compressed” in the synovial fluid, and 18 % of the non-traumatic lesions of the peroneal nerve are said to be caused by intraneural ganglion cysts [93]. Figure 6.12 illustrates these rare findings. Surgical treatment of these intraneural manifestations is quite different if compared to the extraneural ganglia described above (see Sect. 13.3 8) [91].
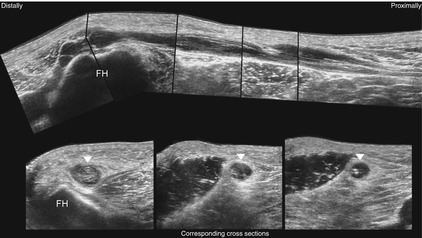

Fig. 6.12
Typical sonographical representation of multiple intraneural ganglion cysts of the common peroneal nerve (arrowheads). FH fibular head. The segmental filling of the nerve segment with synovia is clearly visualized
6.1.8 Application to Polyneuropathies
It has long been known that multifocal palpable nerve enlargements exist in some types of hereditary motor and sensory neuropathy. It is therefore not a surprise that these nerve enlargements can be detected with high resolution ultrasound. This holds true especially for two hereditary neuropathy types, first, the hereditary motor and sensory neuropathy types Ia and Ib and, second, the hereditary neuropathy with liability to pressure palsies. In sonography we find generalized or multifocal nerve enlargements with a focal increase of the cross sectional area, hypoechoic fascicular swelling or even fascicular masking in different peripheral nerves [2, 94, 95] (Fig. 6.11a).
Furthermore, some immune neuropathies show similar alterations: among them are the acute inflammatory demyelinating neuropathy, the chronic inflammatory demyelinating neuropathy (CIDP) and its variants and the multifocal motor neuropathy (see also Sect. 10.5). Typically, not only peripheral nerve segments are involved, but additionally cervical roots and the supraclavicular brachial plexus (Fig. 6.13). If we want to find the differential diagnosis it is important that nerve enlargements are often observed far away from the site of the perhaps expected typical entrapment site, e.g. carpal tunnel, ulnar groove, supinator tunnel. It is interesting that a correlation exists between the sonographically diagnosed segmental nerve enlargements and the conduction blocks registered by electrodiagnostic testing [96–100].
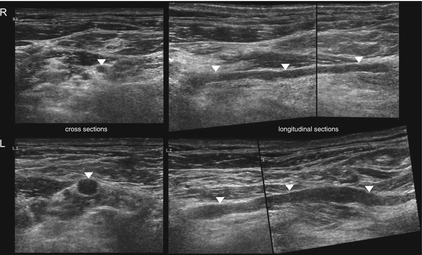

Fig. 6.13
HRUS, segmental enlargement of the root C5 (arrowheads) at the affected side (left) in comparison to the unaffected side (right) in a patient with multifocal motor neuropathy
In a recent report nerve enlargement was commonly diffuse and generally more than twice normal size in subjects with hereditary motor and sensory neuropathy type I (CMT-1) but not in immune neuropathies which showed no, mild or regional nerve enlargement [101]. Several abnormalities have been described regarding further types of polyneuropathies. However, no special characteristics were reported to allow a clear distinction between their different types by means of ultrasound imaging. However, there was evidence that sonographically detectable alterations can be observed before clinical and electrophysiological manifestations occur [100].
6.1.9 Application to Ultrasound Guided Nerve Blocks
In regional anaesthesia, ultrasound guided nerve blocks have been in use for a longer period [102]. It will have to be assessed whether this will further lower the rate of complications which is already very low. In 3% of cases, transient neurological abnormalities occur, and permanent nerve damage is rather rare [103]. In nerves with a high rate of anatomical variability, such as the lateral cutaneous femoral nerve, the success rate of the block can be dramatically increased with ultrasound guidance [104]. Furthermore, less time elapses before onset of the block and its effect lasts longer, the consumption of local anaesthetics is lowered, and finally, the risk of iatrogenic vascular puncture with a damaging hematoma is decreased [105, 106].
The ultrasound guided puncture is applicable in the conservative management of focal painful neuropathies such as meralgia paresthetica or Morton’s metatarsalgia (see Sects. 9.2 and 9.11). Moreover, pain management with ultrasound guided phenol injections in stump neuromas were successfully carried out [107].
6.1.10 Secondary Alterations of the Muscle Following Nerve Damage
Axonal loss leads to a denervation of the muscle substance. In its early stage the reaction is characterized by an oedema of muscles fibers. Ultrasound images reveal them as hyperechoic (Fig. 6.14a). Furthermore, even muscle twitching (fasciculations) can be observed. If no regeneration follows nerve injury muscular atrophy starts. This process is characterized by remodelling of muscle tissue with connective and fatty tissue. The hyperechoic appearance of the affected muscle remains, however, as the muscle diameter decreases [108]. Figure 6.14b illustrates an example.
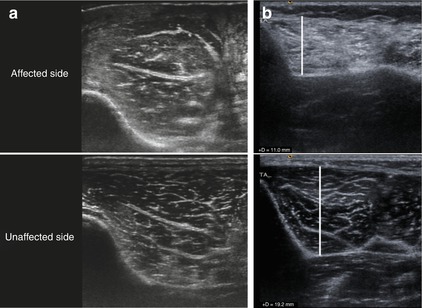

Fig. 6.14
HRUS, secondary alterations of the muscle following nerve damage. (a) Early stage with oedema. (b) Late stage after transformation by connective tissue and decreased muscle diameter (tibialis anterior muscle). Note the hyperechoic appearance in both cases
6.1.11 Conclusions
High resolution ultrasound provides important additional information on the entire aspect of varying occurrences of focal neuropathies, e.g. nerve abnormalities, irritating structures, entrapment dependent on functional limb positions, different intraneural tissue reactions, extra- or intraneural space-occupying lesions, intramuscular reactions and differential-diagnostically important inflammatory diseases. Continuous examination of the entire nerve is possible as well as its dynamic assessment during active and passive limb or muscle movement. There are no side effects and no contraindications. Children can be examined pain free. The method is increasingly more freely available and less cost- and time-consuming than MRI. Limitations only result from deeply situated nerves, obese patients and the phenomenon of acoustic shadowing: osteosynthesis material, hematoma and scar tissue formation can hide small nerve structures from detection. Furthermore, of course, it is not to be neglected that the method depends on the learning curve and the final experience of the examiner. In a very recent study the diagnostic values of MRI vs nerve ultrasound regarding the contribution to the differential diagnosis in mononeuropathies and brachial plexopathies were compared. As a result, ultrasound was valued as more sensitive than MRI (93 % vs 67 %); both techniques yielded an equivalent specificity (86 %) [109]. Additionally, HRUS could better identify multifocal lesions. Therefore authors suggested using HRUS as the preferred initial imaging modality for suspected peripheral nerve pathology [5].
6.2 Magnetic Resonance Imaging (MR Neurography)
6.2.1 Introduction and Technical Notes
While high resolution ultrasound techniques were increasingly applied in the clinical routine, magnetic resonance imaging techniques came to the fore for optimizing the evaluation of peripheral nerve disorders [110, 111]. With the new generation of high-field (3 T) MR scanners and appropriate multichannel phased array surface coils, not only mass lesions of the peripheral nerve sheath but also entrapment neuropathies, peripheral nerve injuries and generalized neuropathies can be evaluated [112, 113]. However, 3 T MR is neither freely available nor cost effective at the moment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







