Intracellular antigen
Associated tumor
Clinical symptoms
Hu (ANNA1)
SCLC
Encephalomyelitis, PCD, LE, brainstem encephalitis
Ri (ANNA2)
Breast, SCLC
Brainstem encephalitis, opsoclonus myoclonus
Yo (PCA1)
Ovary, breast
PCD
CV2 (CRMP5)
SCLC, thymoma
Encephalomyelitis, Chorea, PCD, LE
Amphiphysin
SCLC, breast
SPS, myelopathy and myoclonus, encephalomyelitis
MA-1/2
Testicular seminoma, NSCLC
LE, brainstem encephalitis
PKCγ
Adenocarcinoma
PCD
ARHGAP26
Ovary
PCD
CARPVIII
Ovary, melanoma
PCD
SOX1 (AGNA)
SCLC
LEMS, PCD
ZIC4
SCLC
Cerebellar ataxia
GAD65
–
SPS, cerebellar ataxia, LE
Homer3
–
Cerebellar ataxia
AK5
–
LE
Surface antigens
NMDAR
Ovarian teratoma (58 % in patients >18 years)
Encephalitis
LGI1
LE, tonic seizures
CASPR2
Thymoma (38 %)
LE, Morvan syndrome
AMPAR
SCLC, breast, thymoma (60 %)
LE, psychosis
GABABR
SCLC (50 %)
LE, ataxia
GABAAR
–
Status epilepticus, seizures, encephalitis
mGluR1
M. Hodgkin
Cerebellar ataxia
mGluR5
M. Hodgkin
Ophelia syndrome
DPPX (Kv4.1)
–
Hallucinations, agitation, myoclonia, tremor, seizures, diarrhea
Iglon5
–
NREM/REM parasomnia, sleep apnea, and brainstem dysfunction
GlyR
Lung cancer
PERM, SPS
D2R
–
Basal ganglia encephalitis, Sydenham’s chorea
VGCC
SCLC
LEMS, PCD
DNER (TR)
M. Hodgkin
PCD
12.1.3.1 Antibodies Targeting Intracellular Antigens
All antibodies targeting intracellular antigens are paraneoplastic except anti-GAD65 antibodies, anti-Homer3, and anti-AK5. The detection of these antibodies confirms the immune-mediated origin of the neurological disorder and is helpful in tumor search. The following immunohistochemical staining patterns can be observed:
Hu antibodies (type 1 anti-neuronal nuclear autoantibodies, ANNA1) and Ri antibodies (type 2 anti-neuronal nuclear antibodies, ANNA2) label nuclei and cytoplasm of neurons in cerebrum, cerebellum, and brain stem; nucleoli are spared (Fig. 12.1a). In the peripheral nervous system, Hu-antibodies stain the myenteric plexus, while Ri-antibodies do not. Both antibodies bind to neuron specific RNA-binding proteins with wide distribution in the nervous system (Graus et al. 2001; Pittock et al. 2003). Anti–Yo antibodies (PCA1) label the cytoplasm of Purkinje cells and some stellate and basket cells in the molecular layer of the cerebellum (Fig. 12.1b). The antibodies recognize the cerebellar degeneration–related protein 2 (CDR-2), a Purkinje cell protein that is involved in signal transduction and gene transcription. Anti–CV2 antibodies (CRMP5) label oligodendrocytes in the white matter of cerebellum, brainstem, and spinal cord (Fig. 12.1c). The antibodies target a cytoplasmic protein of the collapsin response mediator protein family with a potential role in synaptic events (Honnorat et al. 1996). Anti–amphiphysin antibodies show a synaptic staining of the molecular and granular cell layer of the cerebellar cortex, and midbrain, the Purkinje cells are unstained (Pittock et al. 2005). Amphiphysin is a synaptic vesicle protein that is important for vesicle membrane recycling after depolarization. Anti–Ma1/2 antibodies show a dot-like staining pattern that is found in the nuclei and cytoplasm of large neurons of the brainstem and hippocampus (Fig. 12.1d). The Ma protein family concentrates in interchromatin granule clusters and coiled bodies in nuclei and cytoplasm and is involved in transcription and pre-mRNA processing (Rosenfeld et al. 2001). Anti–PKCγ (protein kinase Cγ), anti–ARHGAP26 (rhoGTPase-activating protein 26), and anti-CARPVIII antibodies (carbonic anhydrase-related protein VIII) label the cytoplasm, axons, and dendrites of Purkinje cells (Fig. 12.1e). The antibodies recognize the respective intracellular proteins that are all specific for Purkinje cells (Doss et al. 2014; Hoftberger et al. 2013a, 2014). Anti–SOX1 antibodies (anti-glial nuclear antibody, AGNA) label the nuclei of Bergmann glia of the cerebellum (Fig. 12.1f). SOX1 belongs to a family of transcription factors that is expressed in the developing brain (Graus et al. 2005). Anti–ZIC–antibodies (zinc-finger proteins) label the granule cell layer of the cerebellum and less intensively the cytoplasm of Purkinje cells. ZIC proteins play a role in cerebellar development, and the antibodies target a conserved zinc-finger domain that is common in different ZIC proteins (Bataller et al. 2002). Anti–GAD65 antibodies (glutamic acid decarboxylase 65 antibodies) label axonal terminals in the molecular layer, at the base of Purkinje cells, and rosettes of axonal terminals in the glomeruli of the granular layer of the cerebellum (Fig. 12.1g) (Solimena et al. 1990). GAD65 is the brain-specific isoform of GAD and the rate-limiting enzyme for the synthesis of the transmitter gamma-aminobutyric acid (GABA). Anti–Homer3 antibodies show an intense labeling of the molecular layer of the cerebellum and weaker reactivity with the cytoplasm of Purkinje cells. Homer 3 interacts with the metabotropic glutamate receptor type 1 (mGluR1) and enables clustering of the receptor (Hoftberger et al. 2013c). Anti–adenylate kinase 5 (AK5) antibodies react with the cytoplasm of neurons in cerebrum, cerebellum, and brain stem; nuclei are spared. The protein AK5 is neuron specific and involved in metabolic processes and RNA/DNA synthesis (Tuzun et al. 2007). Anti–Tr antibodies label the Purkinje cell cytoplasm and show a dot-like staining pattern in the molecular layer of the cerebellum. The antigen was initially described intracellular but was recently identified as delta/notch-like epidermal growth factor-related receptor (DNER) (De Graaff et al. 2012).
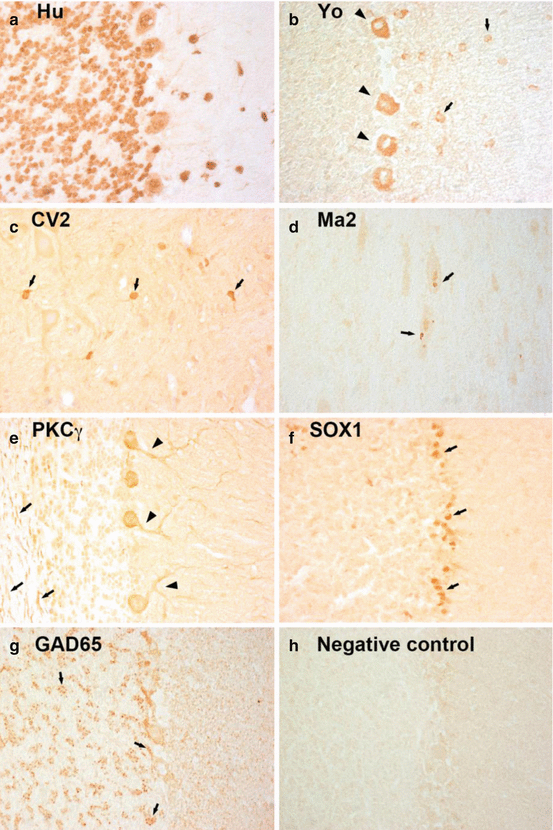

Fig. 12.1
Staining pattern of antibodies targeting intracellular antigens. (a) Anti-Hu-antibodies show an intensive labeling of cytoplasm and nuclei of Purkinje and granule cells. (b) Anti-Yo antibodies label the cytoplasm of Purkinje cells (arrow heads) and stellate and basket cells in the molecular layer (arrows). (c) Anti-CV2-antibodies mark a subgroup of oligodendrocytes in the brainstem (arrows). (d) Anti-Ma2-antibodies show a dot-like staining pattern in large neurons of the brainstem (arrows). (e) Anti-PKC-antibodies label the cytoplasm, axons (arrows), and dendrites (arrow heads) of Purkinje cells. (f) Anti-SOX1-antibodies stain the nuclei of Bergmann glia in the cerebellum (arrows). (g) Anti-GAD65-antibodies show a rosette-like staining pattern in the granular layer of the cerebellum and a dot-like staining of the base of Purkinje cells (arrows). (h) Serum of a healthy individual remains negative. Magnification: (a–h) ×400
12.1.3.2 Antibodies Targeting Surface Antigens
The association of antibodies targeting surface antigens with malignancy is less consistent. The detection of these antibodies is important because patients usually respond to immunotherapy. Both serum and CSF should be tested, and it is reasonable to follow an algorithmic diagnostic approach (Fig. 12.2):
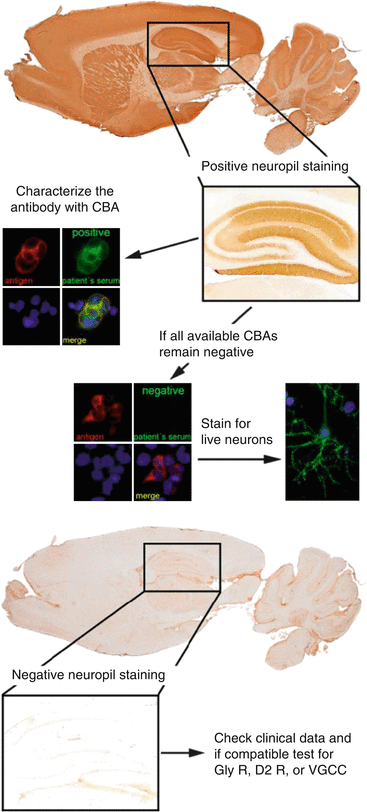

Fig. 12.2
Algorithmic approach for the diagnosis of antibodies targeting surface antigens. Abbreviations: CBA cell-based assay, Gly R Glycine receptor, D2 R Dopamine 2 receptor, VGCC P/Q type calcium channel
A first screening should be performed by a tissue-based assay optimized for surface receptor antibodies, where most of the antibodies show a characteristic neuropil staining pattern in the hippocampus. In case of a positive result, the sample should be tested on a cell-based assay that specifically expresses the antigen of interest (e.g., NMDAR, GABA(B)R, AMPAR). If all currently available cell-based assays remain negative, the sample should be stained on live hippocampal neurons. A positive result confirms that the patient’s antibody recognizes a surface receptor antigen, and the technique can be used for characterizing the novel antibody by immunoprecipitation. Glycinreceptor (GlyR) antibodies, dopamin2 receptor (D2R) antibodies, and P/Q type calcium channel (VGCC) antibodies are not detectable by immunohistochemistry and have to be tested either directly by a cell-based assay (GlyR antibodies, D2R antibodies) or by radioimmunoassay (VGCC antibodies).
12.2 Detection of Anti-glial Antibodies
As mentioned above, various anti-neuronal antibodies have been described in the past few years. In contrast, the role of antibodies to glial antigens for the pathogenesis and diagnosis of inflammatory demyelinating CNS diseases is still unclear, except for antibodies to astrocytic aquaporin-4 (AQP4) in NMO. Evidence for a role of antibodies and B-cells in demyelinating diseases comes from neuropathological investigations (Lassmann et al. 2007), the effect of B-cell directed therapies (Hauser et al. 2008; Keegan et al. 2005; Krumbholz et al. 2012) and the finding of intrathecal immunoglobulin (Ig)G antibody production, dominance of B-cells and oligoclonal IgG bands in the CSF (Cepok et al. 2001; Freedman et al. 2005; Krumbholz et al. 2012; Kuenz et al. 2008; Reindl et al. 2006). Various myelin and non-myelin antigens were suspected as targets for humoral immune reactions in demyelinating diseases (Krumbholz et al. 2012; Reindl et al. 2006). Recently, antibodies to aquaporin-4 (AQP4) have emerged as sensitive and specific biomarkers for NMO (Wingerchuk et al. 2007), and antibodies to the myelin oligodendrocyte glycoprotein (MOG) are associated with a subset of predominantly pediatric demyelinating diseases (Reindl et al. 2013).
12.2.1 Antibodies to Astrocytic Aquaporin-4 (AQP4) in NMO Spectrum Disorders
So far NMO is the only disease among the spectrum of inflammatory demyelinating diseases, which was proven to be antibody mediated. In 2004 Lennon and colleagues identified an autoantibody targeting the astrocytic water channel protein aquaporin-4 (AQP4) as a highly sensitive and specific biomarker for NMO (Lennon et al. 2004), which was included into the diagnostic criteria of NMO (Wingerchuk et al. 2006) and helped to define NMO spectrum disorders (NMOSD) (Wingerchuk et al. 2007). Moreover, recent studies have also confirmed that AQP4-IgG are not only important diagnostic biomarkers but are also relevant in the pathogenesis of NMO by direct transfer of pathology by human AQP4-IgG antibodies to rodents (Bennett et al. 2009; Bradl et al. 2009; Saadoun et al. 2010), thus fulfilling Witebsky’s criteria for autoimmune diseases (Witebsky et al. 1957).
AQP4-IgG antibodies were first discovered by Vanda Lennon using an indirect immunofluorescence assay with a composite substrate of mouse tissue (Lennon et al. 2004). After this discovery, various assays with different sensitivity and specificity have been developed, including tissue-based and cell-based assays (Chan et al. 2010; De Vidi et al. 2011; Fazio et al. 2009; Granieri et al. 2012; Hoftberger et al. 2013b; Iorio et al. 2013; Isobe et al. 2012; Jarius et al. 2007, 2008, 2010b; Jiao et al. 2013; Lennon et al. 2005; Mader et al. 2010; Marignier et al. 2013; Matsuoka et al. 2007; Mckeon et al. 2009; Paul et al. 2007; Pisani et al. 2013; Takahashi et al. 2006, 2007; Waters et al. 2008, 2012). Pictures from tissue- and cell-based assays for AQP4-IgG are shown in Fig. 12.3.
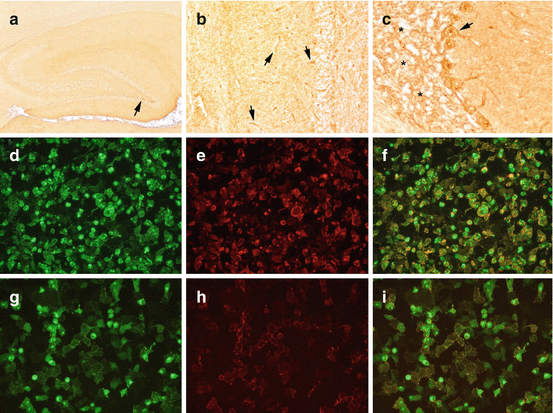

Fig. 12.3
Staining pattern of AQP4 antibodies on a tissue-based assay optimized for surface receptor antibodies. (a) Anti-AQP4-antibodies show a mild neuropil staining pattern in the hippocampus with a stronger laminar staining in the subgranular zone (arrow) and (b) labeling of the glia limitans perivascularis in the entire brain (arrows). (c) The cerebellum shows a marked reticular staining of the granular layer (asterisks) with basket-shaped processes around Purkinje cell bodies (arrow). (d–f) Cell-based assay for AQP4 antibodies. AQP4-EmGFP expressing HEK cells are shown in green (d), bound serum AQP4 IgG antibodies in red (e), and the colocalization of serum autoantibody binding and AQP4-EmGFP expression in yellow (f). (g–i) Cell-based assay for MOG antibodies. MOG-EmGFP expressing HEK cells are shown in green (g), bound serum MOG-IgG antibodies in red (h), and the colocalization of serum autoantibody binding and MOG-EmGFP expression in yellow (i). Magnification: a, ×20; b, ×100; c, ×200; d and 2, ×200
Multicenter studies comparing different assays for AQP4-IgG detection showed the highest sensitivity and specificity for cell-based assays (Waters et al. 2012). Recently, we have compared 21 different AQP4-IgG assays from 15 European laboratories in 101 NMOSD patients and 92 controls for the ERAENET ERARE project EDEN in 2013, and the results indicated that cell-based assays expressing the M23 AQP4 isoform in human cells yielded the highest sensitivity and specificity. Consensus on AQP4-IgG detection method has been established, and cell-based assays should be used for the detection of AQP4 IgG in serum samples. AQP4-IgG are found in 60–95 % of patients who are positive for the diagnostic criteria for NMO (Wingerchuk et al. 2006), and the specificity of the assays used is 90–100 %. Like with most other autoantibodies, the prevalence of AQP4-IgG is higher in female patients (Trebst et al. 2014). Although in most patients AQP4-IgG remain detectable despite immunosuppressive treatment, antibody testing should be performed on samples taken prior to treatment commencement (Jarius et al. 2008; Trebst et al. 2014). According to recent guidelines of the German Neuromyelitis Optica Study Group (NEMOS) AQP4-IgG test results should either be confirmed using a second, methodologically independent assay with high sensitivity and specificity, or testing should be repeated (Trebst et al. 2014).
The diagnostic value of AQP4-Ab in the CSF is controversial because most studies indicate that AQP4-IgG are produced peripherally without clear evidence of intrathecal synthesis (Dujmovic et al. 2011; Jarius et al. 2010a; Takahashi et al. 2007). Only two studies reported the presence of CSF AQP4-IgG in a small number of AQP4-IgG seronegative patients (Klawiter et al. 2009; Long et al. 2013).
12.2.2 Antibodies to the Myelin Oligodendrocyte Glycoprotein (MOG) in Demyelinating Diseases
Antibodies to MOG were extensively analyzed in the past 20 years and results indicated a possible role in MS pathogenesis. Cell-based assays turned out to be of major importance for detecting MOG-specific serum autoantibodies and helped to (re)define the spectrum of MOG autoantibody-associated demyelinating diseases (Reindl et al. 2013). The autoantigen MOG makes up a small part of the myelin sheath and is expressed on the surface of the myelin sheath and plasma membrane of oligodendrocytes (Brunner et al. 1989). In addition MOG is a CNS-specific protein (Brunner et al. 1989), which belongs to the highly conserved immunoglobulin superfamily (Pham-Dinh et al. 1993). The presence of MOG antibodies in CNS demyelinating disease has been controversially described over the last decade of years (Krumbholz et al. 2012; Reindl et al. 2006). This is mainly due to the fact that the detection of conformational dependent anti-MOG antibodies depends on the antigen preparation and detection system. Former studies used Western blot or ELISA assays which do not represent the correct conformation and glycosylation of MOG. A successful detection of conformational MOG-IgG antibodies depends on fluid-phase and cell-based assays. O’Connor and colleagues developed a radioimmunoassay (RIA) and were the first to detect conformational dependent anti-MOG antibodies in a cohort of patients with acute disseminated encephalomyelitis (ADEM) but only rarely in adult onset MS (O’connor et al. 2007). This finding was confirmed by several groups using cell-based assays. Despite the reproducible detection of MOG-IgG antibodies in pediatric ADEM patients, all studies showed a variable degree of MOG-IgG detection in CNS demyelinating diseases and controls (Brilot et al. 2009; Di Pauli et al. 2011; Gredler et al. 2013; Hacohen et al. 2014; Kitley et al. 2012, 2014; Lalive et al. 2011; Mader et al. 2011; Mayer et al. 2013; Mclaughlin et al. 2009; Probstel et al. 2011; Rostasy et al. 2012, 2013; Sato et al. 2014; Selter et al. 2010; Titulaer et al. 2014; Woodhall et al. 2013). The clinical spectrum of MOG-IgG-associated CNS demyelinating diseases is broad, with a higher frequency of MOG-IgG found in pediatric patients with ADEM, CIS, MS, monophasic and recurrent optic neuritis and myelitis, NMO, and patients with NMDAR-encephalitis and demyelination. These differences could be due to variations in methodology, antigen, titer cutoff levels, and age differences of patients, emphasizing the need for a standardized method as well as cutoff values (Reindl et al. 2013). Most groups use a cell-based assay with human MOG transfected cells, and they detect bound antibodies by immunofluorescence or FACS. We have developed a cell-based immunofluorescence assay using living HEK cells transfected with MOG which is labeled with a fluorescence protein on the C terminus (Di Pauli et al. 2011). Like most other groups, we implemented a high titer cutoff value at 1:160 as the antibodies at lower titers can also be found in controls, indicating a more unspecific immune reaction at lower titer levels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






