Author
Patients (n)
Follow-up (years)
Almost pain-free patients (n)
At least ↓ attack frequency >50 % (n)
Complications
17
8.7
6
6
Electrode misplacement (2) or malpositioning (1), infection (4), ICH (1), seizure (1), permanent weakness (1)
Schoenen [43]
6
4
2
1
Fatal ICH (1); panic attack (1); oculomotor disturbances
Starr [47]
4
1
0
2
Oculomotor disturbances, transient loss of consciousness
Owen [40]
1
0.7
1
0
–
Bartsch [2]
6
1.4
2
1
–
11
1
3
3
Oculomotor disturbances (3), transient loss of consciousness (1), micturition syncopes (1)
Seijo [46]
5
2.8
2
3
Euphoria, oculomotor disturbances, headache, increased appetite, cervical dystonia
Total
52
16 (30 %)
16 (30 %)
Technical Aspects, Anatomical Concerns, and Mechanisms of Actions
Surgery may be conducted under general or under local anesthesia which allows a preoperative stimulation to assess eventual side effects, especially gaze disturbances. The use of microelectrode recordings (MER) is not recommended because it does not bring additional information useful to optimize electrode placement and increases the risk of intracerebral hemorrhage (ICH). Common stimulation parameters used for chronic stimulation are frequency 130 Hz, pulse width 60–210, and amplitude 1.5–3.5 V.
The functional target of DBS in CCH is virtual and is consequently defined indirectly using its stereotactic coordinates according to the bi-commissural plan. The most often used target has been initially proposed by Franzini et al. [14] and is located 2 mm lateral to the midline, 3 mm posterior to the mid-commissural point (MCP), and 5 mm below MCP (Fig. 1). A useful anatomic landmark visible on MRI is the anteromedial border of the red nucleus. The neural structure corresponding to these coordinates and whose stimulation induces the therapeutic effect is still debated. An anatomical study of electrode locations has identified several candidate structures [11], including the mesencephalic gray substance and several fascicles connecting the hypothalamus with the autonomic nuclei of the brain stem (Fig. 2). Moreover, the electrode location did not differ between responders and nonresponders, suggesting that other factors not related to electrode misplacement may be responsible for failure of DBS treatment in nonresponders.
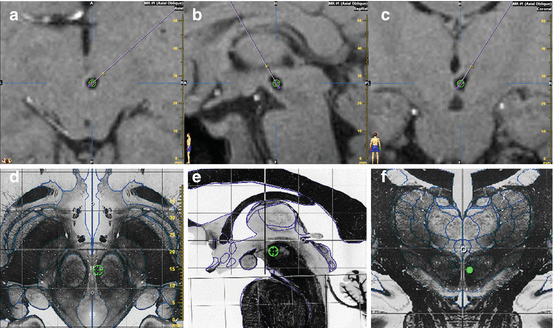
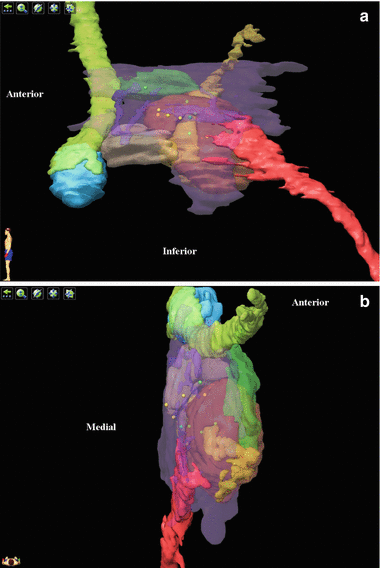

Fig. 1
Postoperative T1-weighted MRI in chronic refractory cluster headache patient treated by deep brain stimulation of the retro-hypothalamic region. Intended stereotactic target (green circle) (x = 2 mm; y = −3 mm; z = −5 mm, relative to the mid-commissural point) is projected within the artifact generated by the electrode on axial (a), sagittal (b), and coronal (c) slices and on the corresponding sections of the Schaltenbrand atlas (d–f)

Fig. 2
Location of DBS electrodes on 3D rendering of relevant anatomic structures of the retro-hypothalamic region, from medial (a) and superior (b) views, in a series of 11 refractory cluster headache patients [11]. Contacts of responders are in green and nonresponders in yellow. The region of interest is centered by the red nucleus (transparent, orange). Medially, the mesencephalic gray substance (transparent, purple) belongs to the wall of the third ventricle and is in continuity with the posterior hypothalamus anteriorly and with the periaqueductal gray substance posteriorly. The mammillary body (light blue), with the mammillothalamic fascicle (light green) and the mammillotegmental fascicle (transparent, dark green), constitutes the macroscopic posterior border of the hypothalamus. The ventral tegmental area (beige) is located immediately posterior to the mammillary bodies. Several bundles cross this area. The fascicle retroflexus of Meynert (yellow, transparent) makes a notch in the medial region of the red nucleus and links the habenula with the interpeduncular nucleus. The medial longitudinal fascicle (red) connects the hypothalamus with autonomic centers in both the brain stem and spinal cord. The dorsal longitudinal fascicle (transparent, purple; only thin portions are individualized at this level) connects the paraventricular nucleus of the hypothalamus with the periaqueductal gray matter, the locus coeruleus, and autonomic centers of the brain stem. Several structures have been erased for simplification
However, two additional neighboring targets seem to be efficient. The first one is actually located in the posterior hypothalamus (4 mm from the third ventricle wall, 2 mm posterior to and 5 mm below the MCP) [46]. Ventriculography-guided implantation of electrode on the floor of the third ventricle via the foramen of Monro was also efficient [3].
Tractography studies confirmed that DBS targets for CCH were located close to fibers connecting the hypothalamus with the brain stem [39, 40]. A positron emission study comparing retro-hypothalamic DBS “on” and “off” conditions showed that the stimulation induced activation in the ipsilateral posterior hypothalamic gray (site of electrode implantation), ipsilateral thalamus, somatosensory cortex and precuneus, anterior cingulate cortex, and ipsilateral trigeminal nucleus and ganglion [33].
Plotted together, these data suggest several putative mechanisms of action for DBS in CCH: (1) inhibition of a CH generator actually located in the hypothalamus and modulated through stimulation of afferent fibers in the retro-hypothalamic area; (2) inhibition of a CH generator located in the retro-hypothalamic region or in the mesencephalic gray substance; and (3) modulation of nonspecific antinociceptive systems, including mesencephalic gray substance or orexinergic system [18].
Complications
Few stimulation-related side effects have been reported: sensation of imminent death, transient loss of consciousness with palsy (stimulation of reticular formation?), micturition syncope, and gaze disturbances (probably related to the stimulation of supranuclear gaze control pathways including rostral interstitial nucleus of medial longitudinal fascicle and interstitial nucleus of Cajal). Two ICH (one death and one permanent neurological deficit) have been reported in early series using MER, probably related to the injury of the paramedian thalamo-peduncular deep penetrating midbrain vessels.
However, considering the high risk of ICH in pioneer DBS studies, some centers rapidly abandoned DBS and opted to a less invasive procedure as occipital nerve stimulation (ONS).
Occipital Nerve Stimulation
The principle of ONS is to deliver a continuous electrical stimulation to the greater occipital nerve (GON) (Fig. 3) and/or to the lesser occipital nerve (LON), via a subcutaneous chronically implanted electrode adjacent to the nerve and connected to a generator. ONS induces paresthesias in the occipital region. Originally described by Weiner [53] to control occipital nerve neuralgia, this technique has been proposed to treat primary headaches, including CCH.
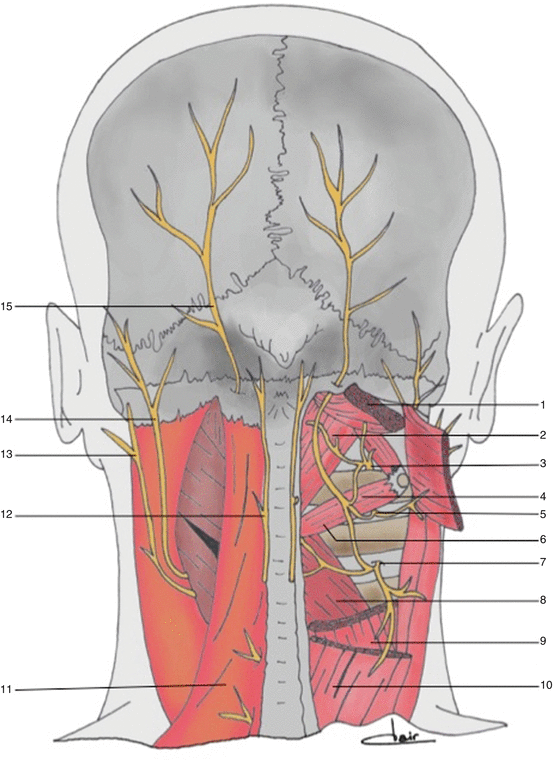

Fig. 3
Anatomy of the occipital and suboccipital regions. 1 Head semispinalis muscle (cut and retracted), 2 rectus posterior muscle of the head, 3 dorsal ramus of C1, 4 head inferior oblique muscle, 5 dorsal ramus of C2, 6 inferior oblique muscle, 7 dorsal ramus of C3, 8 partial section of the head muscle, 9 partial section of the neck muscle, 10 splenius muscle of the head, 11 trapezius muscle, 12 third occipital nerve, 13 great auricular nerve, 14 lesser occipital nerve, 15 greater occipital nerve
Results
ONS for CCH has been studied only in open trials (Table 2) [4–6, 13, 29, 30, 36, 48]. The overall success rate (attack frequency decrease >50 %) was about 75 %, and most of the patients would recommend the operation to a fellow CCH patient. ONS acts like a prophylactic treatment, decreasing the frequency of the CH attacks and their intensity and allowing to decrease the prophylactic drugs in most of the patients, but does not stop the attack once it has begun. Considering their respective risks of complications, ONS should be proposed before DBS in refractory CCH patients.
Table 2
Main studies of occipital nerve stimulation for chronic cluster headache
Study | Patients (n) | Mean follow-up [range] (months) | Recommend the operation | Improved >50 % (n) | CH attack frequency | CH attack intensity | Treatment decreased |
|---|---|---|---|---|---|---|---|
14 | 17.5 [4–35] | 11/14 | 10/14 | −33 % | +8 % | 6/14 | |
15 | 36.8 [11–64] | 10/15 | 12/15 | −94.6 % | – | 4/14 | |
De Quintana et al., 2010 [6] | 4 | 6 [ ] | 4/4 | 4/4 | −56 % | −48.8 % | 3/4 |
Müller, 2011 | 10 | 12 [3–18] | – | 6/10 | −40.3 % | −28.6 % | 3/10 |
Fontaine et al., 2011 [13] | 13 | 14.6 [3–34] | 12/13 | 10/13 | −68.2 % | −48.9 % | 8/13 |
Strand et al., 2011 [48] | 3 | 10 [6–12] | – | 2/3 | −61 % | – | |
Total | 59 | 37/59 (63 %) | 44/59 (75 %) | 24/45 (53 %) |
The feeling of paresthesias appears mandatory to obtain a clinical improvement. Consequently a placebo effect cannot be ruled out, even if its probability is low. However, it will be difficult to show the ONS efficacy in controlled and blinded conditions because the patients perceive ONS-induced paresthesias. Patients who do not feel the paresthesias anymore (lead migration, dysfunction, etc.) often describe a recurrence of their headache attacks within the following days.
ONS has been proposed to treat other medically refractory primary headache, including chronic migraine.
Technical Aspects
The original technique described the implantation of a transverse cylindrical thin electrode crossing the midline from a retro-mastoid incision, allowing to stimulate both sides with one electrode. Multiple variations of this technique have been reported in the literature (Fig. 4), using one or two electrodes, percutaneous cylindrical electrodes or surgical paddle ones, approach from the midline or from one or two retro-mastoid incision(s) [10, 22, 39, 41, 50–53]. Results and complications seemed to be similar whatever the technique was, and no comparative study is available claiming the superiority of one technique over others.
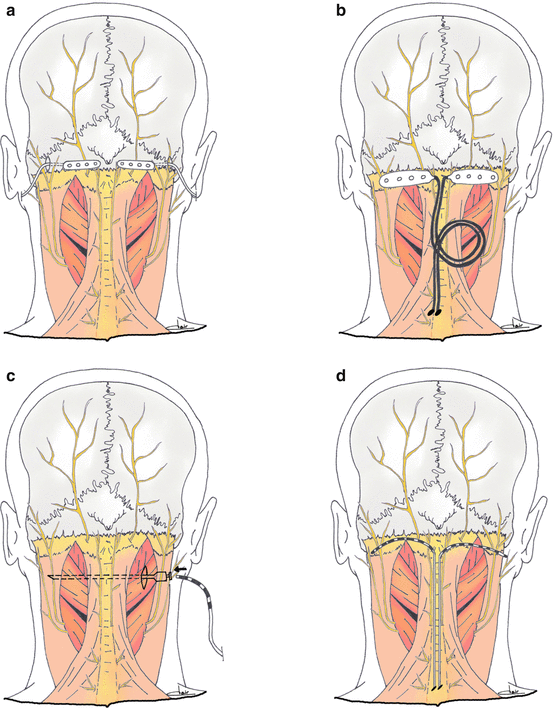
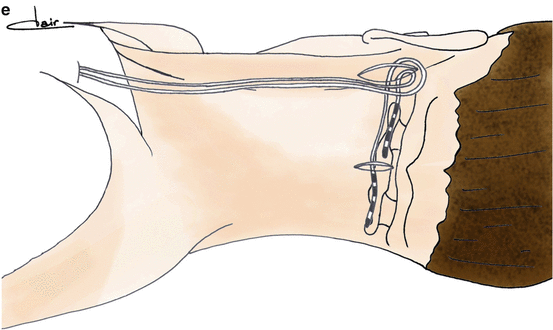


Fig. 4




Examples of different techniques proposed in the literature for bilateral implantation of great occipital nerve stimulation electrodes. Paddle electrodes (a, b) may be implanted using lateral [39] or midline [22] approaches. Cylindrical (c–e) electrodes may be implanted percutaneously from lateral [52, 53] or midline [10] entry points
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








