Fig. 1
Example of how to use HRQOL measures at annual clinic visits. Comparisons of HRQOL and medical data
Finally, if developed using modern measurement techniques (FDA, 2009; Schwarz & Sudman, 1996), PROs can be used as primary or secondary outcomes in clinical trials of new medications or behavioral interventions (Goss & Quittner, 2007; Retsch-Bogart et al., 2009; Turner, Quittner, Parasuraman, Kallich, & Cleeland, 2007). In the context of clinical trials, PROs can also add unique information to physiological outcomes (e.g., pulmonary functioning) and capture both the side effects of new medications and the burden of adhering to a new treatment (Sawicki et al., 2011). For adolescents with chronic conditions, adherence to prescribed medications is quite low, and linking improvement in patient-reported symptoms to their treatment regimen could improve adherence behaviors (Barker & Quittner, 2010; Robinson, Callister, Berry, & Dearing, 2008).
PRO in Clinical Trials
Recently, the Food and Drug Administration (FDA) has recognized the importance of PROs for evaluating new medications and treatments, and has released a “guidance” which outlines criteria for their development and use in clinical trials (FDA, 2009). Currently, 14 % of registered clinical trials report using a PRO; however, this ranges from 5 to 22 % based on the disease (Scoggins & Patrick, 2009). Efforts to develop reliable and valid PROs have been very successful, leading to their use for several different purposes: (1) as primary or secondary outcomes in clinical trials; (2) to evaluate new pharmaceutical, surgical, and behavioral interventions; (3) to describe the impact of illness on patient functioning; (4) to document the natural history of the disease; (5) to analyze the costs and benefits of medical interventions; and (6) to aid in clinical decision-making. Recently, an inhaled antibiotic was approved using a PRO, the Cystic Fibrosis Questionnaire-Revised (CFQ-R; Quittner et al., 2005) as a primary endpoint. Respiratory symptoms improved significantly, with concomitant increases in pulmonary function, as measured by the respiratory symptoms scale of the CFQ-R (McCoy et al., 2008; Oermann et al., 2010; Retsch-Bogart et al., 2009).
In general, the FDA encourages investigators and/or clinicians to determine whether an adequate PRO instrument already exists, because of the intensive effort required to develop and validate a new one (Turner et al., 2007). The FDA reviews every step in the PRO development process, including the adequacy of content validity (e.g., patient interviews, focus groups) and instrument’s psychometric properties. Specific steps for PRO development include (1) hypothesizing a conceptual framework (e.g., linking concepts to product claim); (2) adjusting conceptual framework and drafting a preliminary instrument (e.g., obtaining patient input through qualitative interviews); (3) confirming conceptual framework and other measurement properties (e.g., reliability, validity); (4) collecting, analyzing, and interpreting data (e.g., determining the minimal important difference [MID]); and (5) modifying instrument (see Fig. 2).
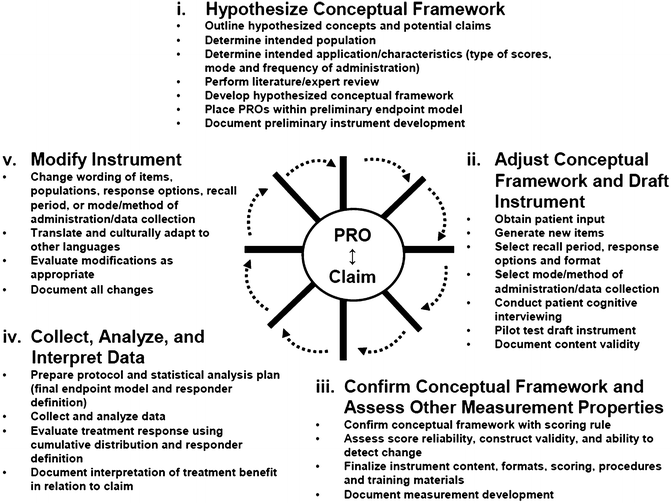

Fig. 2
Development of a PRO Instrument: An Iterative Process, U.S. Department of Health and Human Services, Food and Drug Administration (2009) (Copyright 2009, Food and Drug Administration)
Regardless of the measure’s structure or complexity, to utilize a PRO in a clinical trial, the instrument must meet rigorous psychometric criteria, including documentation of several types of reliability and validity. Reliability indices include internal consistency (Cronbach’s alpha), test–retest reliability, and cross-informant consistency. Construct validity includes predictive (associations between the PRO and other outcomes), convergent (correlations between the PRO and a similar measure), divergent (PRO is not correlated with unrelated constructs), and discriminate (PRO differentiates between patients with differing levels of disease severity). These analyses combine to produce evidence that the “construct” being targeted is measured by the instrument. In addition, the MID, which establishes the smallest change that can be detected by respondents, must be determined (Guyatt, Osoba, Wu, Wyrwich, & Norman, 2002; Quittner, Modi et al., 2009; Wyrwich, Tierney, Babu, Kroenke, & Wolinsky, 2005). The MID provides an empirical method for interpreting the clinical significance of the observed effects (Quittner, Modi et al., 2009).
Health-Related Quality of Life
HRQOL measures are a distinct type of PRO which yield multidimensional profile scores across several areas of functioning. There are both generic and disease-specific HRQOL measures. Generic measures utilize general items that are applicable to both healthy populations and those with chronic conditions. Several generic instruments are well-established, including the Pediatric Quality of Life Inventory (Varni, Seid, & Rode, 1999), the Child Health and Illness Profile (Starfield et al., 1995), and the Youth Quality of Life (Edwards, Huebner, Connell, & Patrick, 2002). These measures have been shown to correlate with disease severity, discriminate between healthy and chronically ill populations, and can be used to compare adolescents with different conditions. However, because they are general, they lack precision and sensitivity to change, and often do not provide specific information for intervention. In addition, these instruments are not accepted by the FDA for approval of new drugs or devices.
In contrast, disease-specific HRQOL measures focus on the domains of functioning most relevant for a particular disease and its treatment. These measures are able to detect small, but clinically meaningful changes and often identify important targets for clinical intervention. Several disease-specific HRQOL measures have been developed for adolescents with chronic diseases including diabetes, obesity, cystic fibrosis, cancer, asthma, epilepsy, and HIV/AIDS (see Table 1). However, disease-specific instruments do not currently exist for a number of conditions, including irritable bowel syndrome (IBS), sickle cell disease, thalassemia, solid organ transplant, and cardiovascular disease, among others. Future research should focus on the development of HRQOL measures in these diseases.
Table 1
Disease-specific health related quality of life measures for adolescents with chronic diseases
Instrument | Age range | Respondent | EBA rating | ePRO |
|---|---|---|---|---|
Diabetes | ||||
Audit of diabetes-dependent QoL—teen | 13–18 years | Adolescent | Well-established | |
Diabetes self-management profile | 6–15 years | Child/adolescent | Well-established | |
Diabetes quality of life measure | 13–17 years | Adolescent | Well-established | |
PedsQL—diabetes module | 5–18 years | Child/adolescent | Well-established | |
2–18 years | Parent | Well-established | ||
Obesity | ||||
Impact of weight on quality of life—kids | 11–19 years | Adolescent | Well-established | |
Sizing them up | 5–18 years | Parent | Approaching | |
Cystic fibrosis | ||||
Cystic fibrosis quality of life questionnaire | 14 years–adult | Adolescent/adult | Well-established | |
Cystic fibrosis questionnaire—revised (CFQ-R) | 6 years–adult | Child/adolescent/adult | Well-established | |
Questions on life satisfaction—cystic fibrosis | 16 years–adult | Adolescent/adult | Well-established | |
Asthma | ||||
About my asthma (AMA) | 6–12 years | Child | Approaching | |
Adolescent asthma quality of life questionnaire (AAQOL) | 12–17 years | Child | Well-established | |
Childhood asthma questionnaires | 4–7 years | Child | Well-established | |
8–11 years | Child/adolescent | Well-established | ||
12–16 years | Adolescent | Well-established | ||
Children’s health survey for asthma (CHSA) | 7–16 years | Child | Well-established | |
5–12 years | Parent | Well-established | ||
Pediatric asthma quality of life questionnaire | 7–17 years | Child/adolescent | Well-established |  |
Pediatric asthma caregiver’s quality of life questionnaire | 7–17 years | Parent | Well-established |  |
Asthma-related quality of life scale (ARQOLS) | 6–13 years | Child | Well-established | |
TACQOL-asthma | 8–16 years | Child and parent | Approaching | |
PedsQL—asthma module | 5–18 years | Child | Well-established | |
2–18 years | Parent | Well-established | ||
Life activities questionnaire for childhood asthma | 5–17 years | Child/adolescent | Approaching | |
How are you? | 8–12 years | Child | Promising | |
8–12 years | Parent | Promising | ||
Integrated therapeutics group child asthma short form | 2–17 years | Parent | Well-established | |
Headaches | ||||
24-h adolescent migraine questionnaire | 12–18 years | Adolescent and parent | Promising | |
PedMIDAS | 6–18 years | Child | Well-established | |
Quality of life headache in youth | 12–18 years | Adolescent and parent | Well-established | |
Epilepsy | ||||
Quality of life in epilepsy inventory for adolescents (QOLIE-AD-48) | 11–17 years | Adolescent | Well-established | |
The child self-report scale and parent-proxy response scale | 8–15+ years | Child/adolescent | Promising | |
6–15 years | Parent | Promising | ||
US version—quality of life in childhood epilepsy questionnaire | 4–18 years | Parent | Approaching | |
Cancer | ||||
Behavioral affective and somatic experiences scale (BASES) | 2–20 years | Child, parent, and nurse | Well-established | |
The Miami pediatric quality of life questionnaire | 1–18 years | Child and parent | Approaching | |
The Minneapolis–Manchester quality of life | 8–12 years | Child/adolescent | Approaching | |
13–18 years | Adolescent | Approaching | ||
The pediatric cancer quality of life inventory (PCQL) | 8–18 years | Child and parent | Well-established | |
The pediatric oncology quality of life scale | 5–17 years | Parent | Well-established | |
PedsQL—cancer module | 8–12 years | Child | Well-established | |
13–18 years | Adolescent | Well-established | ||
Play performance scale for children | 6 months–16 years | Parent | Promising | |
Quality of life—cancer survivors questionnaire | 16–29 years | Adolescent/adult | Well-established | |
The Royal Marsden Hospital pediatric oncology quality of life questionnaire | 3–19 years | Youth and parents | Promising | |
Juvenile rheumatoid arthritis | ||||
Juvenile arthritis quality of life questionnaire | 2–18 years | Child/adolescent | Well-established | |
PedsQL—rheumatoid arthritis module
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||

