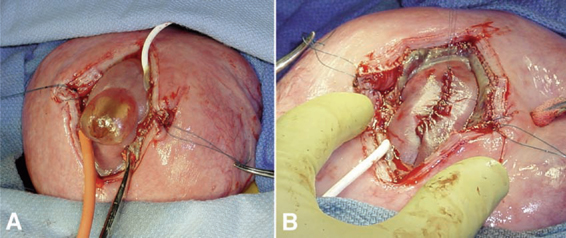
13 In Utero Repair of Myelomeningoceles Nalin Gupta Several diseases can be treated by surgical procedures performed directly on the fetus prior to the anticipated delivery date.1 Most of these diseases, such as congenital diaphragmatic hernia and sacrococcygeal teratoma, are usually fatal if untreated. An open neural tube defect, or myelomeningocele, is different in that fetuses with this condition will usually complete a normal gestation and, if treated after birth, can live for many decades.2 For this reason, the maternal and fetal risk factors of prenatal intervention must be balanced with the potential benefits. Currently, the reported benefits of fetal surgery include a reduction in shunt insertion rates and improvement in hindbrain abnormality.3–5 Published reports of results from fetal myelomeningocele procedures are mostly retrospective case series compared with historical controls. To obtain more conclusive data, the potential benefit of fetal surgery for myelomeningoceles is being examined in a clinical trial directly comparing patients randomized into prenatal and postnatal treatment groups. The neurological deficits caused by a myelomeningocele can be separated into two groups: primary and secondary. The primary neurological deficits are caused by the arrested development of the unclosed neural tube, known as the neural placode, which usually occurs in the lumbosacral region.6 Because neural tube closure occurs during the third and fourth weeks of gestation, the spinal cord in this region is very immature at the stage when a myelomeningocele develops. It is unknown whether the neural placode is capable of further development. It is clear that the normal anatomy of the spinal cord is severely disrupted at the level of the placode.7 The functional neurological level is either at the same level as the vertebral anomaly or actually higher than the vertebral level, resulting in worse neurological function in more than 80% of patients with spina bifida aperta but not with spina bifida occulta.8 There is little that can be done in the postnatal setting to reverse the primary developmental abnormality of the affected spinal cord. Augmenting spinal cord function with tissue grafts or growth factors is speculative at this time. The secondary neurological deficits in patients with spina bifida include delayed loss of motor function, worsening bowel and bladder control, and scoliosis. These symptoms and signs are typically attributed to a symptomatic tethered spinal cord. Magnetic resonance imaging (MRI) studies of most myelomeningocele lesions following repair show a dysplastic spinal cord terminating in the overlying soft tissues at the site of the repaired defect. The presence of the conus at the level of the repair site means that virtually all patients with spina bifida have a tethered spinal cord by radiological criteria. Fortunately, not all patients with spina bifida aperta, or a specific type of spina bifida occulta (e.g., lipomyelomeningocele), will develop true tethered spinal cord syndrome, which consists of the development of clinical signs and symptoms above the area of repair. It is not clear why some patients with a myelomeningocele have either minor or no symptoms of a tethered spinal cord. One possibility is that those patients are likely to maintain normal viscoelasticity of the filum terminale, preventing the lumbosacral cord from unnecessary stretching.9 The theoretical advantage of fetal repair of myelomeningoceles is that the neural tube is covered and protected many months before the expected delivery date. The basis for expecting improved neurological function is that restoration of the dysplastic neural placode within the spinal canal isolates it from the amniotic fluid and prevents ongoing injury.10,11 Additional experimental evidence is derived from experiments performed on fetal sheep. Meuli and others surgically created a spinal cord lesion in fetal sheep at 75 days of gestation that simulated a spontaneous spina bifida lesion.12 After delivery at term, these animals were incontinent and had loss of sensation and motor function below the lesion level. The gross and microscopic appearance of the exposed spinal cord resembled a human spina bifida lesion. Animals with surgically created spina bifida lesions were then treated using a myocutaneous flap at 100 days of gestation. These animals were then carried to full-term gestation and had near-normal motor function and normal bowel and bladder control. The results of these experiments suggest that early repair of an exposed spinal cord may preserve neurological function and may allow improvement through plasticity.13 Although provocative and interesting, these large-animal experiments clearly rely on a model system that does not have all the features of the human disease. Performing fetal surgery for myelomeningoceles would be expected to have the best results if performed as early as possible. However, this is limited by the timing of diagnosis and technical limitations of the actual surgical procedure. Most myelomeningoceles are detected during the second trimester, either to investigate a positive maternal screening test or during a routine ultrasound study. The quality of current ultrasonography allows detection of most fetuses with myelomeningoceles by the midportion of the second trimester.14 From a practical viewpoint, this means that a diagnosis is made between 18 and 22 weeks of gestation. Taking into consideration current obstetrical practice, it is unlikely that detection of fetuses with spina bifida will occur any earlier unless new, more sensitive screening tests are discovered. Fetal surgery cannot be performed without the participation of a well-trained team. Successful completion of a procedure requires specialized maternal and fetal anesthesia, the ability to perform uterine opening (hysterotomy) and closure with control of uterine contractions, and continuous intraoperative fetal monitoring. These techniques are described elsewhere and will not be addressed further in this chapter.15 The goals of the fetal procedure are similar to the standard postnatal procedure. They include identification of the neural placode, separation of the placode from the surrounding epithelium, closure of the dura and overlying soft tissues, and closure of the skin. These goals must be modified during a fetal procedure by several limitations. The first limitation is the tenuous nature of the fetal tissues. The neural placode is extremely fragile, and even limited manipulation leads to loss of tissue integrity. Although the nerve roots are able to withstand some handling, excessive tension causes avulsion. The dura is often insubstantial, is transparent when mobilized, and has the characteristics of arachnoid in older children. The skin is able to handle surgical dissection, but excessive tension leads to tearing. A second limitation is the inability to properly place the fetus in a neutral position at all times during the procedure. The location of the hysterotomy is determined in part by the position of the fetus but also the location of the placenta. The orientation of the fetus can be confirmed prior to hysterotomy with intraoperative ultrasound, but at times it is difficult to maintain the lumbar spine in a horizontal position, which interferes with the closure of the lesion. The neural placode is more visible in the fetus than in the term infant. The edges of the placode are continuous with the arachnoid, which is extremely thin and translucent. If the myelomeningocele sac is intact, the placode will be lifted upward away from the surface of the back (Fig. 13.1A). The epithelium of the skin does not usually reach the edge of the placode. The clear identification of the intervening arachnoid usually allows the placode to be divided from its attachments with sharp dissection. Depending upon the consistency of the placode, the neural tube can be retubularized; however, if the placode is particularly fragile, this step may not be possible. The dura is loosely attached to the underlying subcutaneous tissues just lateral to the spinal canal. After incising the dura at its lateral junction with the dermis, gentle instillation of saline into the epidural plane lifts the dura away from the underlying tissues, which minimizes the manipulation of the dura. Between 18 and 20 weeks of gestation, the dura can be very thin and difficult to handle. After 22 weeks of gestation, the dura becomes more substantial and can be handled more easily.
Rationale for Surgery
Timing for Surgery
Surgical Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







