Urinary incontinence (UI) is a very common complaint among the elderly and adversely affects the lives of millions of people. The prevalence of UI is 5% to 30% of people living in the community, 40% to 70% of elderly patients in an acute hospital setting, and up to 50% of people living in nursing homes (
41). Currently, the health care cost of UI exceeds $20 billion annually in the United States (
33). The total lifetime costs for treatment of UI in one older adult have been estimated at $60,000 (
10).
Age-related changes of bladder function play a significant role in increasing UI. The aging bladder demonstrates increases in uninhibited detrusor contractions, impaired contractility, abnormal detrusor relaxation patterns, and reduced capacity. In addition, the aging population experiences a shift in nocturnal diuresis, and the frequency of nocturia increases (
69). The aging male population can suffer from benign prostatic hyperplasia (BPH) with secondary bladder changes. BPH is evident in 50% of the male population by age 50 and 80% by age 80 (
13). Beyond primary changes to the genitourinary tract, elderly adults can suffer from other risk factors that contribute to UI. These include decreased mobility and manual dexterity, difficulties accessing toilets, impaired mentation, and a variety of comorbid medical conditions and multiple medications outlined in
Table 16-1.
Initial evaluation of elderly patients with UI should include a comprehensive history and physical exam including a thorough “brown bag review” of all medications used by the patient. The workup should include a comprehensive physical examination, a rapid screening for cognition using the Mini-Mental State Examination, neurologic exam, and evaluation of the patient’s mobility and manual dexterity. Useful diagnostic tests include urinalysis, urine culture, urine cytology, free flow and postvoid residual by catheter or ultrasound, 72-hour voiding diary, cough stress test, and urodynamics if empirical treatment has failed. Combining information gleaned from these tests can allow identification of the cause and contributing factors leading to the UI and can help the health care team to formulate and initiate an appropriate treatment plan.
BLADDER INNERVATION
An understanding of bladder innervation and physiology is required to diagnose and treat UI. The lower urinary tract is innervated by three sets of peripheral nerves involving the parasympathetic, sympathetic, and somatic nervous systems. Pelvic parasympathetic nerves arise from S2-4 of the spinal cord in a region termed the sacral parasympathetic nucleus (SPN); they
send axons through the ventral roots to peripheral ganglia in the detrusor wall where they release acetylcholine. This action facilitates voiding by excitation of the detrusor muscle and relaxation of the urethra. Lumbar sympathetic fibers arising from the lumbar spinal cord follow a path through the sympathetic chain ganglion to the inferior mesenteric ganglia and then through the hypogastric nerve to pelvic ganglia. These fibers provide noradrenergic excitatory and inhibitory input, which relaxes the detrusor and contracts the sphincteric mechanism to facilitate urine storage. Finally, somatic influences are exerted via the pudendal nerve; motoneurons to the external urethral sphincter (EUS) arise from the lateral border of the ventral horn, known as the Onuf nucleus, and contract the EUS (
16).
Afferent pathways through the pelvic, hypogastric, and pudendal nerves transmit information from the lower urinary tract to the lumbosacral spinal cord. Pelvic nerve afferents monitor the volume of the bladder and amplitude of bladder contractions via myelinated A-δ and unmyelinated C axons. A-δ fibers located in the bladder smooth muscle sense bladder fullness (i.e., wall tension) and serve as low-threshold mechanoreceptors. C fibers located in the mucosa respond to both stretch (i.e., bladder volume sensors) or nociception and overdistention. C fibers are largely insensitive to normal distention. They become mechanosensitive after activation by a chemical mediator during inflammation (
16).
REFLEX CIRCUIT
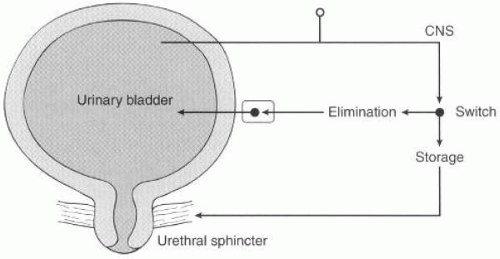
The lower urinary tract has two major functions: (a) low-pressure bladder filling and storage of urine with continence and (b) low-pressure periodic voluntary emptying. The micturition cycle is organized via a simple on-off switching circuit (
Fig. 16-1) that maintains a reciprocal relationship between the urinary bladder and the urethral outlet (
16). During bladder filling and urine storage, three functions must occur: (a) the bladder must continue to maintain low pressure and accommodate an increasing volume of urine, (b) the bladder outlet must remain closed, and (c) there should be an absence of abnormal bladder contractions. In contrast, during bladder emptying/voiding three things must happen: (a) the bladder must contract for an adequate magnitude and duration, (b) a concomitant lowering of resistance at the level of the smooth and striated sphincter must occur, and (c) there must be an absence of anatomic obstruction (
67).
Voiding dysfunction may occur from damage to the micturition reflex at the storage level or at the elimination level. Thus, voiding dysfunction may be categorized as the failure to store urine or the failure to empty urine secondary to the bladder or the outlet (
Table 16-2). In addition, a mixture of both of these classifications can exist. By extrapolating from a basic switchlike framework, many different etiologic possibilities that affect storage, emptying, or both can be defined as illustrated in
Table 16-3.
STORAGE AND ELIMINATION PHASES OF THE BLADDER
By viewing micturition as a simple on-off circuit, adding the complexity of the afferent and efferent neural control of the urinary tract yields a complex but understandable circuit, as described below (
16). Intravesical pressure measurements during bladder
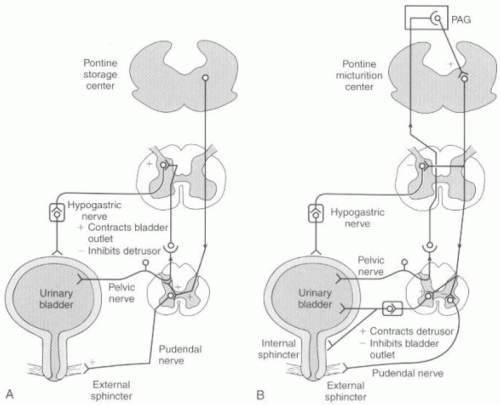
filling should show constant low pressure and low levels of afferent pelvic nerve activity. The responding efferent pathways produce pudendal nerve outflow and contraction of the EUS, sympathetic nerve stimulation causing detrusor inhibition with activation of the continence mechanism, and finally, inactivation of the sacral parasympathetic outflow (
Fig. 16-2A).
The initiation of micturition can be activated either voluntarily or involuntarily. The activation of micturition incites high levels of afferent pelvic nerve activity, which stimulates the brainstem micturition center. The pontine micturition center (PMC) then inhibits somatic nerve output (pudendal nerve) to cause relaxation of the EUS and inhibits sympathetic outflow (hypogastric nerve) to cause a release of inhibition on the relaxation of bladder and contraction of the urethra. The PMC stimulates parasympathetic outflow, which excites the bladder and relaxes the internal sphincter smooth muscle of the urethra. Voiding occurs by initial relaxation of the urethral sphincter, and then a few seconds later, contraction of the bladder, increase in bladder pressure, and flow of urine.
Maintenance of the voiding reflex is through ascending afferent input from the spinal cord, which may pass through the periaqueductal gray matter (PAG) before reaching the PMC (
Fig. 16-2B).
PATHOPHYSIOLOGY OF VOIDING DYSFUNCTION
Dysfunction at any level of the urinary mechanism outlined in the previous section can cause loss in the regulation of voiding and result in UI. Loss of detrusor inhibition and relaxation of the internal sphincter occur, which results in uninhibited bladder contractile activity, causing urgency and UI. Injuries to the PMC or to the descending spinal pathways cause a loss of coordination between the bladder and the urinary sphincter, which results in an uninhibited contraction and a nonrelaxing urinary sphincter (detrusor sphincteric dyssynergia). This causes a high-pressure, poor emptying bladder, which eventually can decompensate and cause upper urinary tract damage. This type of dysfunction is seen with dementia, stroke, multiple sclerosis (MS), tumors, or other brain injuries. Exceptions to this framework are lesions of the internal capsule (typically strokes), which result in uninhibited and poorly sustained detrusor contractions and inadequate voiding pressure with sphincteric dysfunction.
Injuries at the level of the spinal cord can be from trauma, disk herniation, vascular lesions, MS, tumors, syringomyelia, myelitis, or iatrogenic causes. In the acute phase, they lead to a hypocontractile bladder with low pressure and large bladder capacity. Over time, these injuries lead to a loss of innervation and sphincteric spasticity and voiding dyssynergia. Bladder wall fibrosis occurs and can result in detrusor hypertrophy, high voiding pressure, ureteral reflux or obstruction leading to renal damage, and UI. Cervical spinal cord injuries may cause autonomic dysreflexia. Injuries above the level of the sympathetic outflow from the cord result in hypertensive blood pressure fluctuations, bradycardia, and sweating with stimulation of the lower urinary tract.
Pathologic lesions that occur at or below the sacral micturition center (S2-4) may be caused by spinal cord injury, damage to the anterior horn cells from poliovirus or herpes zoster, or iatrogenic causes such as radiation or surgery. These lesions are often incomplete and cause a mixture of overactive bladder (OAB) activity with weakened muscle contractility. Sphincter tone is diminished, and bladder pressure is low, but capacity is high and can lead to UI. Voiding is accomplished through straining.
Hypocontractile bladders can result from other neurologic conditions including diabetes mellitus, tabes dorsalis, pernicious anemia, and posterior spinal cord lesions. The pathology is not damage to the detrusor muscle nucleus, but rather a loss of sensory input through the afferent feedback pathway. Eventually, this may lead to a loss of neurotransmission in the dorsal horn of the cord and loss of perception in bladder filling, resulting in overstretching of the detrusor. An atonic detrusor with a large volume capacity and high residual urine may eventually occur.
Pelvic trauma may injure the nerves to the sphincter, and selective denervation can lead to an incompetent sphincter mechanism and UI. Peripheral nerve injuries can also occur with radical pelvic surgeries or radiation therapy. During pelvic surgeries, damage to the peripheral nerves may result in a bladder that cannot accommodate with filling; the smooth muscle is intact, but no central reflex organizes muscle activity, and a hypertonic bladder wall with high-pressure filling and poor storage of urine can occur. Radiation can result in denervation of the detrusor or sphincter. At the detrusor level, it can cause fibrosis and loss of compliance of the detrusor, with failure to both store and empty urine adequately.
Incomplete neural lesions can cause variable lower urinary tract dysfunction that is often not predictable based on the level of the injury. In disease processes such as MS, the neural lesions can be present at multiple levels and can confuse clinical presentations in patients (
16,
39,
63,
67).