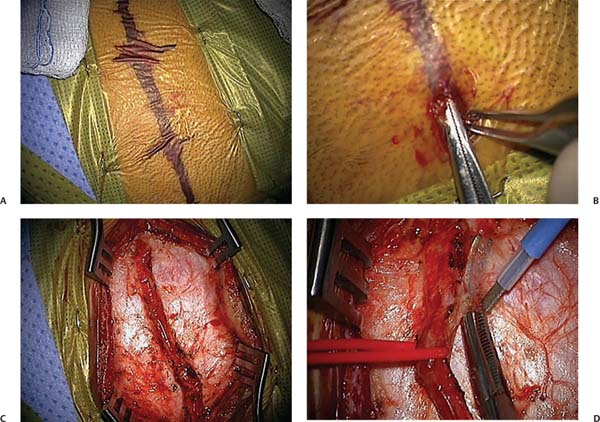
Chapter 10 Moyamoya syndrome is an increasingly recognized cause of stroke characterized by progressive stenosis of the intracranial internal carotid arteries (ICAs) and their proximal branches. As these vessels narrow, a network of collateral vessels develops near the carotid bifurcation, on the cortical surface, and also from branches of the external carotid artery (ECA). In rare cases, this process may involve the posterior circulation, including the basilar and posterior cerebral arteries. Some authors have distinguished between moyamoya disease—the idiopathic form of moyamoya—and moyamoya syndrome—defined as the vasculopathy found in association with other conditions. These conditions include, but are not limited to, prior radiotherapy to the head or neck for tumors, Down syndrome, neurofibromatosis type I, large facial hemangiomas, sickle cell disease; autoimmune disorders such as Graves disease, congenital cardiac disease, and renal artery stenosis. Most patients present with ischemic symptoms; recurrent transient ischemic attacks (TIAs) or strokes. Adults also commonly present with intraparenchymal hemorrhage. Seizures and headaches have been described as presenting symptoms in a minority of patients. The natural history of moyamoya is not well known. Disease progression can be slow with rare, intermittent strokes, or fulminant, with rapid neurologic decline.1,2 However, moyamoya inevitably progresses in the majority of patients—including asymptomatic individuals—and medical therapy alone does not halt the process.3–5 Two-thirds of patients with moyamoya have symptomatic progression over a 5-year period with poor outcomes if left untreated.6–8 This number contrasts strikingly with an estimated rate of only 2.6% of symptomatic progression following surgical treatment in a meta-analysis of over 1100 patients.9 The workup of a patient in whom the diagnosis of moyamoya syndrome is suspected typically begins with either a magnetic resonance imaging (MRI) study or computed tomography (CT) of the brain. Findings include narrowed ICAs, middle cerebral arteries (MCAs), and anterior cerebral arteries (ACAs) in association with moyamoya collaterals in the basal ganglia and frequently evidence of previous infarcts. Sometimes reduced cerebral blood flow can be inferred from fluid-attenuated inversion recovery (FLAIR) images with the so-called ivy sign, a bright signal in cortical sulci. Definitive diagnosis is based on a distinct arteriographic appearance characterized by bilateral stenosis of the distal intracranial ICA extending to the proximal ACA and MCA (Fig. 10.1). Disease severity is frequently classified into one of six progressive stages originally defined by Suzuki et al.3 Development of an extensive collateral network at the base of the brain along with the classic “puff of smoke” appearance on angiography is seen during the intermediate stages of the Suzuki grading system. External carotid imaging is essential to identify preexisting collateral vessels so that surgery, if performed, will not disrupt them. Aneurysms or arteriovenous malformations, known to be associated with some cases of moyamoya, can also be best detected by conventional angiography. Once diagnosed, patients should be referred to a specialized center experienced with the treatment of moyamoya. In general, neurologic status at time of treatment, more so than the age of the patient, predicts long-term outcome.1 As such, early diagnosis of moyamoya is of paramount importance and needs to be coupled with expeditious institution of therapy. The curious fact that the arteriopathy of moyamoya involves the ICA, while sparing the ECA provides the foundation underlying surgical treatment of moyamoya, which is predicated on utilizing the ECA to provide arterial supply to the ischemic hemisphere. Two general methods are employed: direct and indirect. Fig. 10.1 The initial steps in performing a pial synangiosis. (A) Course of the parietal branch of the superficial temporal artery (STA) marked out following Doppler mapping. (B) Distal incision with subcutaneous dissection using fine S-curved snap. (C) Completed dissection of the STA branch prior to freeing up an adventitial cuff. (D) Elevation of the STA with an associated adventitial cuff, using the monopolar cautery. Note that all of the surgery occurs under the microscope. In direct revascularization, a branch of the ECA (usually the superficial temporal artery [STA]) is divided and anastomosed to a cortical artery (usually a distal branch of the MCA)—an STA-MCA bypass. In contrast, indirect techniques involve mobilizing vascularized tissue supplied by the ECA (dura, muscle, pedicles of the STA) and placing it in contact with the brain; facilitating ingrowth of new vessels to the cortex. Historically, direct procedures have been used in adults, with immediate increase of blood flow to the ischemic brain cited as a major benefit. Augmentation of cerebral blood flow usually does not occur for several weeks with indirect techniques. However, direct bypass is often technically difficult to perform in children because of the small size of donor and recipient vessels, making indirect techniques appealing. Nonetheless, direct operations have been successful in children as have indirect procedures in adults.10–12 Considerable debate exists regarding the relative merits and shortcomings of the two approaches with some centers advocating combinations of both.12–14 Here we review a variant of the indirect approach, pial synangiosis used successfully in both children and adults first described by the senior author, including indications, peri-operative management, surgical technique, and strategies for complication avoidance.1 We have recently published a specific perioperative protocol for sickle cell patients with moyamoya (Table 10.1).15 This protocol—absent the hematology-related interventions—has been adapted from our practice for all patients with moyamoya and highlights general strategies we have found useful in the surgical management of this condition.
Indirect Revascularization for Moyamoya Syndrome
 Pial Synangiosis
Pial Synangiosis
One day before surgery |
Continue aspirin therapy (usually 81 mg once a day orally if <70 kg, 325 mg once a day orally if 70 kg or more) Admit patient to hospital for overnight intravenous hydration (isotonic fluids 1.25–1.5 times maintenance) |
At induction of anesthesia |
Institute EEG monitoring Maintain normotension during induction; also normothermia (especially with smaller children), normocarbia (avoid hyperventilation to minimize cerebral vasoconstriction, pCO2 >35 mm Hg), and normal pH Place additional intravenous lines, arterial line, Foley catheter, and pulse oximeter Place precordial Doppler to monitor for venous air emboli (relevant with thicker bone resulting from extramedullary hematopoiesis). |
During surgery |
Maintain normotension, normocarbia, normal pH, adequate oxygenation, normothermia, and adequate hydration EEG slowing may respond to incremental blood pressure increases or other maneuvers to improve cerebral blood flow. |
Postoperatively |
Avoid hyperventilation (relevant in crying children); pain control is important Maintain aspirin therapy on postoperative day 1 Maintain intravenous hydration at 1.25–1.5 times maintenance until child is fully recovered and drinking well (usually 48–72 hours) |
Source: Revised from Smith ER, McClain CD, Heeney M, Scott RM. Pial synangiosis in patients with moyamoya syndrome and sickle cell anemia: perioperative management and surgical outcome. Neurosurg Focus 2009;26(4):E10.
 Indications for Surgery
Indications for Surgery
In the setting of patients with radiographically confirmed moyamoya syndrome, surgery is indicated in the cases with the following:
 History of neurologic symptoms due to apparent cerebral ischemia
History of neurologic symptoms due to apparent cerebral ischemia
 Cerebral circulation and metabolism studies indicating deficiencies in regional cerebral blood flow, vascular response, and/or perfusion reserve (helpful but not mandatory)
Cerebral circulation and metabolism studies indicating deficiencies in regional cerebral blood flow, vascular response, and/or perfusion reserve (helpful but not mandatory)
Surgery is relatively contraindicated in patients who are a poor operative risk (severe cardiac disease, advanced debilitation from stroke burden, or other severe comorbidities). In addition, patients with an unclear diagnosis or who have individual hemispheres with a low Suzuki grade (I or—rarely—II) are sometimes observed closely with serial imaging before committing to surgery.
 Preoperative Strategy and Imaging
Preoperative Strategy and Imaging
Preoperative management of moyamoya patients is critical to the success of surgery. Strategy is based on the use of appropriate imaging for planning and the maintenance of hypervolemia, normocarbia, and prevention of thrombosis. A full six-vessel (both ICAs, both ECAs, and both vertebrals) diagnostic angiogram is critical to the planning of the procedure for
 Accurate identification of disease status
Accurate identification of disease status
 Identification of transdural collaterals so that they may be preserved during surgery
Identification of transdural collaterals so that they may be preserved during surgery
 Confirmation of the presence of a suitable donor scalp vessel (usually the parietal branch of the STA)
Confirmation of the presence of a suitable donor scalp vessel (usually the parietal branch of the STA)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree